|
|
| | Chloro(1,5-cyclooctadiene)rhodium(I) dimer Basic information | | Reaction |
| Product Name: | Chloro(1,5-cyclooctadiene)rhodium(I) dimer | | Synonyms: | Bis(1,5-cyclohexadiene)-mu,mu'-dichlorodirhodium;Bis(cycloocta-1,5-diene)dichlorodirhodium;Chloro(1,5-cyclooctadiene)rhodium dimer;Dichloro-bis-1,5-cyclooctadiene dirhodium;Di-mu-chlorobis(1,5-cyclooctadiene)dirhodium;di-mu-chlorobis[(1,2,5,6-eta)-1,5-cyclooctadiene]di-rhodiu;Rhodium 1,5-cyclooctadiene chloride dimer;Rhodium chloro(cycloocta-1,5-diene) dimer | | CAS: | 12092-47-6 | | MF: | C16H24Cl2Rh2 | | MW: | 493.08 | | EINECS: | 235-157-6 | | Product Categories: | Morpholines/Thiomorpholines ,Isoxazoles;Rh;Rh (Rhodium) Compounds;Catalysts for Organic Synthesis;Classes of Metal Compounds;Homogeneous Catalysts;Metal Complexes;Synthetic Organic Chemistry;Transition Metal Compounds;organometallic complexes | | Mol File: | 12092-47-6.mol | 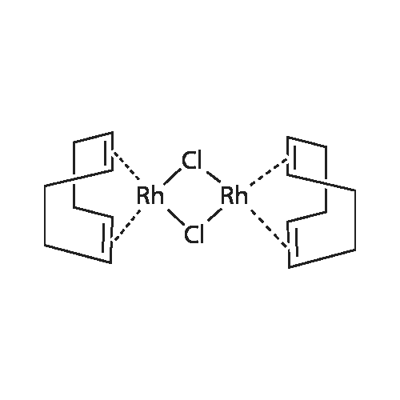 |
| | Chloro(1,5-cyclooctadiene)rhodium(I) dimer Chemical Properties |
| Melting point | 243 °C (dec.) (lit.) | | density | 1.94[at 20℃] | | vapor pressure | 0Pa at 25℃ | | storage temp. | Inert atmosphere,Room Temperature | | solubility | Soluble in chloroform, dichloromethane, and methanol. Sparingly soluble in most common solvents. | | form | Crystals or Crystalline Powder | | color | Orange to orange-brown | | Water Solubility | 1.56g/L at 20℃ | | Sensitive | Air Sensitive & Hygroscopic | | Exposure limits | ACGIH: TWA 0.01 mg/m3; TWA 1 mg/m3
NIOSH: IDLH 2 mg/m3; IDLH 100 mg/m3; TWA 0.001 mg/m3; TWA 0.1 mg/m3 | | Stability: | hygroscopic | | LogP | 3.16 at 20℃ | | NIST Chemistry Reference | Bis(1,5-cyclooctadienerhodium chloride)(12092-47-6) | | EPA Substance Registry System | Rhodium, di-.mu.-chlorobis[(1,2,5,6-.eta.)-1,5-cyclooctadiene]di- (12092-47-6) |
| Hazard Codes | Xi | | Risk Statements | 36/37/38 | | Safety Statements | 26-37/39 | | WGK Germany | 3 | | F | 3 | | TSCA | Yes | | HS Code | 28439000 |
| | Chloro(1,5-cyclooctadiene)rhodium(I) dimer Usage And Synthesis |
| Reaction |
- Catalyst for coupling 1,3-dienes with activate methylene compounds.
- Rhodium source for various asymmetric hydrogenation systems and asymmetric hydrosilylation of ketones.
- Rhodium source for asymmetric reductive aldol reaction.
- Cis-hydroboration of terminal alkynes.
- Rhodium source for [5 + 2] additions.
- Highly enantioselective for [2+2+2] carbocyclization reactions.
- Enantioselective hydroboration of cyclopropenes.
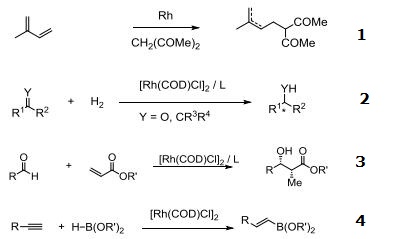
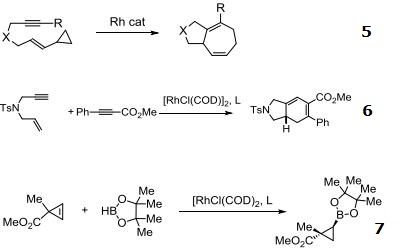
| | Chemical Properties | orange crystals | | Uses | It is a widely used precursor to homogeneous catalysts. This is a chiral catalyst capable of asymmetrically hydrogenating certain prochiral alkenes. Chloro(1,5-cyclooctadiene)rhodium(I) dimer is also used in the synthesis of other metal ligands for use in catalysis. | | Uses | Chloro(1,5-cyclooctadiene)rhodium(I) dimer ([Rh(COD)Cl]2) can be:
- Employed for the synthesis of rhodium complex of heterocyclic carbenes (NHCs).
- Modified and coated on the surface of ferrite magnetic nanoparticles for catalyzing hydroformylation reaction of olefins.
- Used for the synthesis of triple-layer structure to be used as a ring-opening polymerization catalyst.
- As an effective catalyst for dehydrogenation of amine-borane adducts (hydrogen storage materials) such as ammonia-borane.
| | General Description | This product has been enhanced for catalytic efficiency. | | Flammability and Explosibility | Not classified | | reaction suitability | core: rhodium
reagent type: catalyst |
| | Chloro(1,5-cyclooctadiene)rhodium(I) dimer Preparation Products And Raw materials |
|