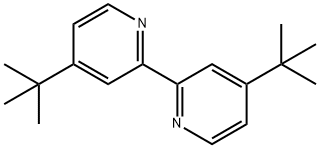
4,4'-Di-tert-butyl-2,2'-bipyridine--A useful ligand in chemical reactions
Mar 24,2020
Physical and Chemical Properties
4,4'-di-tert-butyl-2,2'-dipyridine is white crystals, with melting point of 159-161℃(lit.), boiling point of 395.4±42.0 ℃. It is hygroscopic substance.
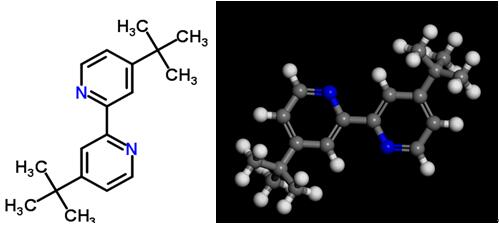
Fig 1. Chemical structure formula and three-dimensional structure of 4,4'-Di-tert-butyl-2,2'-bipyridine
Application
As ligand
The interaction of vanadium(III) chloride (VCl3) with 4,4′-di-tert-butyl-2,2′-bipyridine (dbbpy) in air resulted in the monomeric oxidovanadium(IV) complex [VOCl2(dbbpy)(H2O)] (1) in high yield. The octahedral oxidovanadium(IV) complex, [VOCl2(dbbpy)(H2O)] (1), containing the 4,4′-di-tert-butyl-2,2′-bipyridine (dbbpy) ligand was synthesized and characterized using X-ray diffraction, IR and EPR spectroscopies. This complex was tested as a catalyst for epoxidation of cyclooctene with TBHP in chloroform. A conclusion to be drawn from this study is that one-electron processes occur in the presence of the vanadium(IV) precatalyst, the oxidant and chloroform, generating radical species. In addition, using chloroform in combination with the TBHP oxidant leads to the formation of trans-1,2-dichlorocyclooctane as an unwanted side product of 1,2-dichlorination. It therefore seems wise to avoid the use of chloroform as a solvent in catalyzed oxidations with TBHP (and likely also with H2O2), at least with a vanadium(IV) precatalyst[1].
The dinuclear complex [Mo2O6(di-tBu-bipy)2] (1) (di-tBu-bipy = 4,4′-di-tert-butyl-2,2′-bipyridine) was obtained as a minor product of the hydrothermal reaction of MoO3 and di-tBu-bipy, and structurally characterized by single-crystal X-ray diffraction.A rare example of a dinuclear molybdenum complex exhibiting the {Mo2O6}0 core has been isolated from the hydrothermal reaction of MoO3 and 4,4′-di-tert-butyl-2,2′-bipyridine, and structurally characterized by single crystal X-ray diffraction. The complex is a particularly active and regioselective catalyst for the epoxidation of non-functionalized olefins using tert-butylhydroperoxide as oxidant.To conclude, the dioxo-bridged molybdenum(VI) complex described in this communication represents a new member of the increasingly large family of oxomolybdenum complexes containing the ligand 4,4′-di-tert-butyl-2,2′-bipyridine. Within this family complex 1 stands out as being a particularly active and regioselective catalyst for the epoxidation of non-functionalized olefins[2].
A new palladium catalyzed synthesis of 4-cyano-1,10-phenanthroline (nphen, 1), 4,7-dicyano-1,10-phenanthroline (dnphen, 2) and their corresponding ruthenium complexes. The two cationic complexes, [(tbbpy)2Ru(nphen)]2+ 3 and [(tbbpy)2Ru(dnphen)]2+ 4 (tbbpy = 4,4′-di-tert-butyl-2,2′-bipyridine), were synthesized using an improved microwave procedure[3].
The ground- and excited-state structures for a series of Os(II) diimine complexes [Os(N∧N)(CO)2I2] (N∧N = 2,2′-bipyridine (bpy) (1), 4,4′-di-tert-butyl-2,2′-bipyridine (dbubpy) (2), and 4,4′-dichlorine-2,2′-bipyridine (dclbpy) (3)) were optimized by the MP2 and CIS methods, respectively. The spectroscopic properties in dichloromethane solution were predicted at the time-dependent density functional theory (TD-DFT, B3LYP) level associated with the PCM solvent effect model. It was shown that the lowest-energy absorptions at 488, 469 and 539 nm for 1–3, respectively, were attributed to the admixture of the [dxy (Os) → π∗(bpy)] (metal-to-ligand charge transfer, MLCT) and [p(I) → π∗(bpy)] (interligand charge transfer, LLCT) transitions; their lowest-energy phosphorescent emissions at 610, 537 and 687 nm also have the 3MLCT/3LLCT transition characters. These results agree well with the experimental reports. The present investigation revealed that the variation of the substituents from H → t-Bu → Cl on the bipyridine ligand changes the emission energies by altering the energy level of HOMO and LUMO but does not change the transition natures[4].
References
[1] Iakov S. Fomenko, Sandrine Vincendeau, Eric Manoury, Rinaldo Poli, Pavel A. Abramov, Vladimir A. Nadolinny, Maxim N. Sokolov, Artem L. Gushchin,An oxidovanadium(IV) complex with 4,4′-di-tert-butyl-2,2′-bipyridine ligand: Synthesis, structure and catalyzed cyclooctene epoxidation,Polyhedron,Volume 177,2020.
[2] Tatiana R. Amarante, Patrícia Neves, Filipe A. Almeida Paz, Martyn Pillinger, Anabela A. Valente, Isabel S. Gonçalves,A dinuclear oxomolybdenum(VI) complex, [Mo2O6(4,4′-di-tert-butyl-2,2′-bipyridine)2], displaying the {MoO2(μ-O)2MoO2}0 core, and its use as a catalyst in olefin epoxidation,Inorganic Chemistry Communications,Volume 20,2012,Pages 147-152.
[3] Robert Staehle, Roberto Menzel, Katrin Peuntinger, T. David Pilz, Frank W. Heinemann, Dirk M. Guldi, Rainer Beckert, Sven Rau,An improved synthesis of 4-cyano-1,10-phenanthroline, 4,7-dicyano-1,10-phenanthroline and their bis(4,4′-di-tert-butyl-2,2′-bipyridine)ruthenium(II) complexes,Polyhedron,Volume 73,2014,Pages 30-36.
[4] Yu-Hui Wu, Qing-Jiang Pan, Xin Zhou, Bao-Hui Xia, Tao Liu, Hong-Xing Zhang,Theoretical studies on the structural and optical properties of a series of Os(II) diimine complexes [Os(N∧N)(CO)2I2] (N∧N=2,2′-bipyridine(bpy), 4,4′-di-tert-butyl-2,2′-bipyridine(dbubpy), 4,4′-dichlorine-2,2′-bipyridine(dclbpy)),Journal of Molecular Structure: THEOCHEM,Volume 860, Issues 1–3,2008,Pages 111-118.
- Related articles
- Related Qustion
Germanium [symbol Ge; Chemical Abstracts Service Registry Number (CASRN): 7440-56-4] is never found in a pure state in the environment. In the refined state, it is a gray– white metalloid.....
Mar 6,2020Inorganic chemistryDibutyl phthalate is a colorless transparent oily liquid with a slight aromatic odor. It is insoluble in water, soluble in common organic solvents and hydrocarbons. It is obtained by using phthalic anhydride and n-butanol as raw materials.....
Mar 24,2020Catalyst and Auxiliary4,4'-Di-tert-butyl-2,2'-dipyridyl
72914-19-3You may like
- Property and application of 11,12-Dihydroindolo[2,3-a]carbazole
Sep 11, 2023
- What is Ectoine?
Apr 12, 2022
- What is 4-Dimethylaminopyridine?
Jun 30, 2020
4,4'-Di-tert-butyl-2,2'-dipyridyl manufacturers
- 4,4'-Di-tert-butyl-2,2'-bipyridine
-
- $23.00 / 5g
- 2024-07-22
- CAS:72914-19-3
- Min. Order: 1g
- Purity: 0.98
- Supply Ability: 10kg
- 4,4'-Di-tert-butyl-2,2'-bipyridine
-
- $10.00 / 1g
- 2024-04-15
- CAS: 72914-19-3
- Min. Order: 5g
- Purity: 98%
- Supply Ability: 200kgs
- 4,4'-Di-tert-butyl-2,2'-bipyridine
-
- $1.10 / 1g
- 2021-10-29
- CAS:72914-19-3
- Min. Order: 1g
- Purity: 99.00%
- Supply Ability: 100 Tons Min