Boc-(R)-3-amino-4-(2,4,5-trifluoro-phenyl)-butyric acid: properties, synthesis and applications
Oct 13,2023
General Description
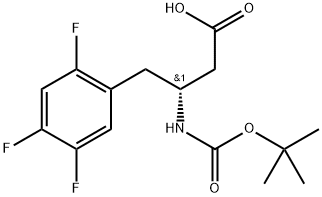
Boc-(R)-3-amino-4-(2,4,5-trifluoro-phenyl)-butyric acid is a solid compound used as an intermediate for the synthesis of sitagliptin, a medication for treating type 2 diabetes. It has a predicted pKa of 4.30±0.10, a melting point range of 136-138℃, and a predicted boiling point of 443.1±45.0℃. The compound is slightly soluble in chloroform, DMSO, ethyl acetate, and methanol. It should be stored between 2-8°C. The synthesis involves a multi-step process, including the preparation of Wittig reagent and Hoffmann rearrangement. Sitagliptin, the final product, is a dipeptidase-IV (DPP-4) inhibitor that helps regulate blood glucose levels by increasing insulin secretion and decreasing glucagon release. Overall, Boc-(R)-3-amino-4-(2,4,5-trifluoro-phenyl)-butyric acid plays a crucial role in the synthesis of sitagliptin and offers significant benefits for managing diabetes.

Figure 1. Boc-(R)-3-amino-4-(2,4,5-trifluoro-phenyl)-butyric acid
Properties
Boc-(R)-3-amino-4-(2,4,5-trifluoro-phenyl)-butyric acid is a solid compound with white-to-off-white color and a predicted pKa of 4.30±0.10. It has a melting point range of 136-138℃ and a predicted boiling point of 443.1±45.0℃. The compound has a density of 1.292±0.06 g/cm3, according to predictions. It should be stored between 2-8°C. In terms of solubility, Boc-(R)-3-amino-4-(2,4,5-trifluoro-phenyl)-butyric acid is slightly soluble in chloroform, DMSO, ethyl acetate, and methanol. The compound is commonly used in the pharmaceutical industry as an intermediate for the synthesis of sitagliptin, a widely used drug for the treatment of type 2 diabetes. Overall, the properties of Boc-(R)-3-amino-4-(2,4,5-trifluoro-phenyl)-butyric acid make it a suitable reactant for medicinal chemistry applications. Its solubility in common solvents and its stability under storage conditions make it a useful starting material for the synthesis of complex pharmaceuticals. 1
Synthesis
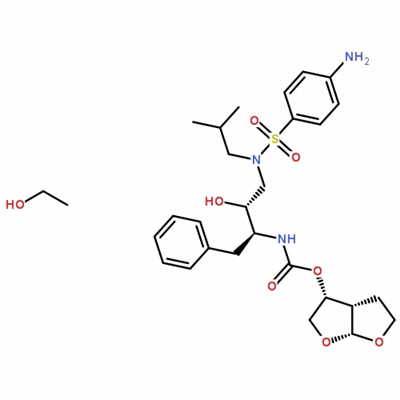
Boc-(R)-3-amino-4-(2,4,5-trifluoro-phenyl)-butyric acid is a critical intermediate in the synthesis of sitagliptin, a drug used to treat type 2 diabetes. A multi-step retrosynthetic analysis was conducted to develop an efficient synthesis route for this compound. The synthesis began by envisioning chiral amido acid 7 as the precursor for the chiral β-amino center, which was planned to be synthesized through asymmetric hydrogenation of the monoester of itaconic acid derivative 4c, followed by amidation. However, attempts to synthesize the required starting materials through Stobbe condensation failed, and a new route involving the preparation of Wittig reagent 6 and subsequent reaction with 2,4,5-trifluorobenzaldehyde 1 resulted in the desired product in high yields. The chiral monoester 5 obtained from this synthesis was then converted to the corresponding amide for Hoffmann rearrangement, which involved overcoming challenges in amidation reactions with ammonia. Calcium chloride was found to provide the best results, and various reagents were examined for the Hofmann rearrangement. It was discovered that the use of bromine in combination with Hunig's base or DBU in tert-butanol resulted in high enantioselectivity but low yield due to evaporation. The development of a novel synthesis route for Boc-(R)-3-amino-4-(2,4,5-trifluoro-phenyl)-butyric acid utilized commercially available starting materials and incorporated stereoselective catalytic asymmetric reduction and Hoffmann rearrangement techniques to install the chiral β-amino center necessary for the production of sitagliptin. 2

Figure 2. Synthesis of (R)-3-(tert-butoxycarbonylamino)-4-(2,4,5-trifluoro phenyl) butanoic acid
Applications
Boc-(R)-3-amino-4-(2,4,5-trifluoro-phenyl)-butyric acid is a crucial intermediate in the synthesis of sitagliptin, a medication used for the treatment of type 2 diabetes. Sitagliptin phosphate, the first dipeptidase-IV (DPP-4) inhibitor approved by the FDA in 2006, has demonstrated significant hypoglycemic effects when used alone or in combination with other antidiabetic drugs like metformin and pioglitazone. It is known to be safe, well-tolerated, and associated with minimal adverse reactions. The application of Boc-(R)-3-amino-4-(2,4,5-trifluoro-phenyl)-butyric acid lies in its role as a reactant for the preparation of DPP-4 inhibitors, including sitagliptin, which effectively regulate blood glucose levels in individuals with type 2 diabetes. These inhibitors work by inhibiting the enzymatic activity of DPP-4, an enzyme responsible for the degradation of incretin hormones that play a crucial role in glucose homeostasis. By inhibiting DPP-4, sitagliptin helps to increase insulin secretion and decrease glucagon release, ultimately leading to improved glycemic control. In conclusion, Boc-(R)-3-amino-4-(2,4,5-trifluoro-phenyl)-butyric acid is an essential intermediate in the synthesis of sitagliptin, a widely used medication for the treatment of type 2 diabetes, offering significant benefits in terms of blood glucose management and minimal side effects. 3
Reference
1. PubChem. COMPOUND SUMMARY: Boc-(R)-3-Amino-4-(2,4,5-trifluorophenyl) butanoic acid. National Library of Medicine, 2006, CID: 7146288.
2. Sreenivasulu K, Chaudhari PS, Achanta S, Sud A, Dahanukar V, Cobley CJ, Llewellyn-Beard F, Bandichhor R. Synthesis of (R)-3-(tert-Butoxycarbonylamino)-4-(2,4,5-trifluorophenyl)butanoic Acid, a Key Intermediate, and the Formal Synthesis of Sitagliptin Phosphate. Chem Asian J. 2020 May 15;15(10):1605-1608.
3. Kim GH, Jeon H, Khobragade TP, Patil MD, Sung S, Yoon S, Won Y, Choi IS, Yun H. Enzymatic synthesis of sitagliptin intermediate using a novel ω-transaminase. Enzyme Microb Technol. 2019 Jan;120:52-60.
- Related articles
- Related Qustion
- What is 3-(Boc-amino)-4-(2,4,5-trifluorophenyl)butyric acid? Sep 2, 2021
(R)-3-((tert-Butoxycarbonyl)amino)-4-(2,4,5-trifluorophenyl)butanoic Acid has been used as a reactant for the preparation of dipeptidyl peptidase-4 (DPP4) inhibitors for the treatment of type 2 diabetes, such as sitagliptin.
Salmeterol can improve asthma control and lung functionby causing bronchodilation and inhibiting inflammatory responses in the airways, with a favorable safety profile.....
Oct 13,2023APIDarunavir ethanolate: the drug approved for the treatment of HIV and also hypothesised to inhibit the protease of SARS-CoV-2.....
Oct 13,2023DrugsYou may like
Boc-(R)-3-Amino-4-(2,4,5-trifluorophenyl)butanoic acid manufacturers
- Boc-(R)-3-Amino-4-(2,4,5-trifluorophenyl)butanoic acid
-

- $0.00 / 1kg
- 2025-04-23
- CAS:486460-00-8
- Min. Order: 1kg
- Purity: 98% up by HPLC
- Supply Ability: 20tons
- BOC-(R)-3-AMINO-4-(2,4,5-TRIFLUORO-PHENYL)-BUTYRIC ACID
-

- $10.00 / 1KG
- 2025-04-23
- CAS:486460-00-8
- Min. Order: 1KG
- Purity: 99%
- Supply Ability: 10 mt
- Boc-(R)-3-Amino-4-(2,4,5-trifluorophenyl)butanoic acid
-

- $0.00 / 1kg
- 2025-04-21
- CAS:486460-00-8
- Min. Order: 1kg
- Purity: 98
- Supply Ability: 1000