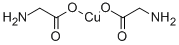Copper citrate or glycinate: Which is better?
Jun 7,2024
Copper(II) citrate, also known as cuprocitrol, is an ionic compound of copper and citric acid with the formula Cu3(C6H5O7)2 or Cu3C12H10O14, with a molecular weight of 568.85 g/mol. It exists as a seafoam green hemipentahydrate and a sky-blue anhydrous solid. Copper citrate is applied to treat wine to remove fermentation and storage-related off-flavours (sulphide off-flavours, flavours caused by reduction reactions, flavours caused by hydrosulfuric acid and mercaptans). The copper sulphide formed during the treatment precipitates in wine as it is a poorly soluble compound and can be separated by filtration.

Copper citrate is the most common type of dietary copper used on the market. This substance is cheap to mass-produce, but some concerns about its bioavailability exist. Copper glycinate, however, is bonded to a glycerin substrate and absorbed directly into the bloodstream. This type of copper has much higher bioavailability than copper citrate, but scientists are not sure how much better the absorption of copper bis-glycinate might be yet.
Some individuals prefer citrate forms of minerals because of the number of studies on this mineral chelate; others look to avoid citrates as some individuals can have trouble tolerating them (this is rare).
According to certain nutritionists, individuals with certain absorption conditions might have trouble absorbing copper citrate supplements. Like all metallic trace nutrients, copper is naturally hard to absorb. Hence, it's necessary to take copper in food or mix it with certain chemicals or materials to make it bioavailable.
While there isn't a lot of definitive data on this subject, the nutrition world believes that copper glycinate is the way of the future. Even in the absence of a scientific consensus, people are abandoning copper citrate and choosing copper bis-glycinate because this new kind of copper supplement is guaranteed to absorb even if your gut lining isn't at its best. Some consider t'ha'tCopper glycin chelates are probably more bioavailable due to higher stability in the stomach, intestine and in the presence of absorption inhibitors (like other bivalent ions, oxalate, phytate, etc.) when taken with food or other dietary supplements.
- Related articles
- Related Qustion
- The application of Copper glycinate in the feed industry Nov 30, 2023
A practical application of Copper glycinate is as a source of dietary copper in animal feeds. In addition, Cu chelate with amino acid or peptide (organic Cu) is a potential substitute for CuSO4 in the feed industry.
Copper glycinate
13479-54-4You may like
Copper glycinate manufacturers
- Copper Glycinate
-

- $0.00 / 20kg
- 2025-04-02
- CAS:13479-54-4
- Min. Order: 20kg
- Purity: 98%
- Supply Ability: 2000
- copper diglycinate
-

- $0.00 / 20kg
- 2025-04-02
- CAS:13479-54-4
- Min. Order: 20kg
- Purity: 98%
- Supply Ability: 2000
- GLYCINE CUPRIC SALT
-

- $0.00 / 10kg
- 2025-04-02
- CAS:13479-54-4
- Min. Order: 20kg
- Purity: 98%
- Supply Ability: 2000mt