Efficacy and safety of Diazoxide
Feb 16,2022
General description
Diazoxide is a benzothiadiazine derivative that is a peripheral vasodilator used for hypertensive emergencies. It lacks diuretic effect, apparently because it lacks a sulfonamide group.
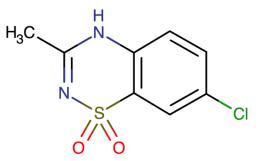
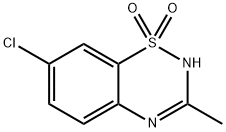
Figure 1 the molecular formula of diazoxide
Hyperinsulinemic hypoglycemia (HH) describes the condition and effects of low blood glucose caused by excessive insulin. Many cases are reported in childhood as a congenital disorder [1]. Congenital hyperinsulinemia (CHI) is the most common and serious cause of persistent hypoglycemia in newborns and children. It occurs between 1:2500 and 50,000 live births [2]. CHI is characterized by excessive secretion of insulin by pancreatic B cells, which is the most common cause of persistent hypoglycemia in infancy [2]. CHI is a heterogeneous disease in clinical manifestations, imaging, histology, and genetics. So far, mutations in more than 10 different genes (ABCC8, KCNJ11, GLUD1, GCK, HADH, HK1, CACNA1D, FOXA2, UCP2, SLC16A1, HNF4A, HNF1A, PMM2, and PGM1) have been reported in the genetic etiology of CHI [3]. CHI has three main histological types: focal, diffuse, and atypical [4].
Diazoxide is the first choice for treating CHI. It is a nondiuretic benzothiadiazine originally formulated as a peripheral vasodilator to reduce severe hypertension by smooth muscle relaxation [5]. It was first used to treat CHI in the 1960s [6] and has been the primary treatment since then. Diazoxide is the only drug approved for this indication in the United States, Canada, the United Kingdom, the European Union, China, Australasia, and Japan, [7]. Diazoxide acts by binding to the sulfonylurea receptor-1 subunit in the ATP-sensitive K+ (KATP) channel, causing the channel to open and increase its permeability to potassium ions. This results in the excessive polarization of beta cells, followed by the inhibition of Ca2+-dependent insulin secretion [8].
Commercial brand-proglycem
PROGLYCEM® (diazoxide) (9) is a nondiuretic benzothiadiazine derivative taken orally for the management of symptomatic hypoglycemia. PROGLYCEM® Capsules contain 50 mg diazoxide, USP. The Suspension contains 50 mg of diazoxide, USP in each milliliter and has a chocolate-mint flavor; alcohol content is approximately 7.25%. Other ingredients: Sorbitol solution, chocolate cream flavor, propylene glycol, magnesium aluminum silicate, carboxymethycellulose sodium, mint flavor, sodium benzoate, methylparaben, poloxamer 188, propylparaben, and purified water. Hydrochloric acid or sodium hydroxide may be added to adjust pH.

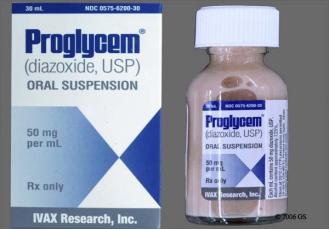
Figure 2 The oral suspension of diazoxide.
Indications and administration
It is used parentally to treat hypertensive emergencies. Also used to treat hypoglycemia secondary to insulinoma. For infants and Children: Leucine sensitivity, islet cell hyperplasia, nesidioblastosis, extrapancreatic malignancy, islet cell adenoma, or adenomatosis. PROGLYCEM® may be used preoperatively as a temporary measure, and postoperatively, if hypoglycemia persists (9).
The usual daily dosage is 3 to 8 mg/kg, divided into two or three equal doses every 8 or 12 hours. In certain instances, patients with refractory hypoglycemia may require higher dosages. Ordinarily, an appropriate starting dosage is 3 mg/kg/day, divided into three equal doses every 8 hours. Thus, an average adult would receive a starting dosage of approximately 200 mg daily (9).
Mechanism of action
Diazoxide is a potassium channel activator. Its mechanism of action revolves around enhancing cell membrane permeability to potassium ions. This action consequently elicits the relaxation of local smooth muscles. This switches off voltage-gated calcium ion channels which inhibits the generation of an action potential. Diazoxide inhibits insulin release from the pancreas, by opening potassium channels in the beta cell membrane (1). Diazoxide is chemically related to thiazide diuretics but does not inhibit carbonic anhydrase and does not have chloriuretic or natriuretic activity (1). It also exhibits hypotensive activity by reducing arteriolar smooth muscle and vascular resistance (1-2).
Efficacy and safety
Several clinical studies investigated the efficacy and safety of diazoxide for treating CHI (10–15). However, the results remain ambiguous. Meissner et al. (10) reported that 47/114 (41.2%) patients responded to diazoxide. Hu et al. (11) found that 36/44 (81.8%) patients were responsive to diazoxide treatment.
Chen et al (16), found that 71% (646/831) of patients responded to diazoxide treatment. Besides, about 25% of children with CHI were partially or completely unresponsive to diazoxide. Other drugs, such as octreotide, may be required for the second-line treatment in such children.
The most common side effect is mild-to-severe hypertrichosis, which is thought to depend on the dose for each patient [15]. In this study, the most common adverse drug reaction was hypertrichosis (45%). For older children, body hair can be troublesome, and they may choose to use other drugs to avoid this complication. In theory, local KATP channel blockers, such as toluene butylamine, may reduce hair growth, but this indication has not been systematically assessed. The retention of sodium and fluorine is a common side effect of diazoxide (18%) (14). All patients taking diazoxide should be carefully monitored for weight, electrolytes and edema, and cardiopulmonary function, especially when starting or increasing doses. The simultaneous use of diuretics, such as chlorothiazide, can reduce urinary retention. In 2015, the US Food and Drug Administration issued a drug safety statement because 11 infants treated with nitrous oxide developed pulmonary hypertension (PH). It is believed that about 2.4% of all children treated with diazoxide have PH (14,15). These data were consistent with the results of the present study (2%). A recent study published by Herrera et al. (14) employed all patients with a formal diagnosis of hyperinsulinemia. Their study included perinatal stress−induced hyperinsulinemia and showed that 2.4% of patients developed PH after starting the use of sodium diazoxide. They believed that PH is more likely to occur in preterm and low-birth-weight infants.
References
1. Hasegawa T, Tanaka T, Kanzaki S, Sugihara S, Yakotani S, Tanaka H. Diagnosis and therapeutic guideline of hyperinsulinemic hypoglycemia. J Japan Pediatr Soc. 2006;110:1472–4.
2. Yorifuji T, Masue M, Nishibori H. Congenital hyperinsulinism: Global and J apanese perspectives. Pediatrics International. 2014;56(4):467–76. pmid:24865345
3. Flanagan SE, Kapoor RR, Hussain K, editors. Genetics of congenital hyperinsulinemic hypoglycemia. Seminars in pediatric surgery; 2011: Elsevier.
4. Demirbilek H, Hussain K. Congenital Hyperinsulinism: Diagnosis and Treatment Update. J Clin Res Pediatr Endocrinol. 2017;9(Suppl 2):69–87.
5. De Cosio AP, Thornton P. Current and Emerging Agents for the Treatment of Hypoglycemia in Patients with Congenital Hyperinsulinism. Pediatric Drugs. 2019:1–14. pmid:30604241
6. Drash A, Kenny F, Field J, Blizzard R, Langs H, Wolff F. The therapeutic application of diazoxide in pediatric hypoglycemic states. Annals of the New York Academy of Sciences. 1968;150(2):337–55.
7. von Oettingen J, Armstrong K, Raza J, Raskin J, Zacharin M, Chanoine J-P. Application for inclusion of Diazoxide in the WHO Model List of Essential Medicines for Children (March 2019).
8. Hansen JB. Towards selective Kir6. 2/SUR1 potassium channel openers, medicinal chemistry and therapeutic perspectives. Current medicinal chemistry. 2006;13(4):361–76.
9. https://www.rxlist.com/proglycem-drug.htm#description
10. Meissner T, Wendel U, Burgard P, Schaetzle S, Mayatepek E. Long-term follow-up of 114 patients with congenital hyperinsulinism. European journal of endocrinology. 2003;149(1):43–51
11. Hu S, Xu Z, Yan J, Liu M, Sun B, Li W, et al. The treatment effect of diazoxide on 44 patients with congenital hyperinsulinism. Journal of Pediatric Endocrinology and Metabolism. 2012;25(11–12):1119–
12. Wang W-Y, Sun Y, Zhao W-T, Wu T, Wang L, Yuan T-M, et al. Congenital hyperinsulinism in China: a review of Chinese literature over the past 15 years. Journal of clinical research in pediatric endocrinology. 2017;9(3):194.
13. Fukutomi M, Shimodera M, Maeda Y, Iwakura M, Hara M. Safety and effectiveness, including intelligence prognosis, of diazoxide in pediatric patients with hyperinsulinemic hypoglycemia: special survey in Japan (long-term, all-case survey). Clinical Pediatric Endocrinology. 2018;27(3):131–43.
14. Herrera A, Vajravelu ME, Givler S, Mitteer L, Avitabile CM, Lord K, et al. Prevalence of adverse events in children with congenital hyperinsulinism treated with diazoxide. The Journal of Clinical Endocrinology & Metabolism. 2018;103(12):4365–72.
15. Thornton P, Truong L, Reynolds C, Hamby T, Nedrelow J. Rate of serious adverse events associated with diazoxide treatment of patients with hyperinsulinism. Hormone research in paediatrics. 2019;91(1):25–32.
16. Chen X, Feng L, Yao H, et al. Efficacy and safety of diazoxide for treating hyperinsulinemic hypoglycemia: A systematic review and meta-analysis[J]. PLoS ONE, 2021, 16(2):e0246463.
- Related articles
- Related Qustion
- Uses of Diazoxide Jan 17, 2022
Diazoxide is a well-characterized low molecular weight (MW 230.7) drug that is a derivative of benzothiadiazine but that possesses antidiuretic effects. It has been approved and used since the 1970s for treating hypertension and hypoglycemi
- Diazoxide: Pharmacologic action, Side effects and Uses Oct 23, 2019
Diazoxide is an anti-hypertensive drug having a basic structure of thiazide drug, but has no diuretic effects.
DDQ is a deprotection agent for ketals, acetals, and thioacetals. It is a useful electron transfer reagent for synthesis of quinolones and an oxidizing agent used to synthesize steroids.....
Feb 16,2022Catalyst and AuxiliaryD-mannose (or mannose) is a type of sugar found in a number of fruits and vegetables, including cranberries, black and red currants, peaches, green beans, cabbage, and tomatoes. It's also produced in the body from glucose, another form of s....
Feb 16,2022Biochemical EngineeringDIAZOXIDE
364-98-7You may like
- Diazoxide
-

- $0.00 / 1g
- 2025-01-13
- CAS:364-98-7
- Min. Order: 1g
- Purity: More Than 99%
- Supply Ability: 50kg/Month
- Diazoxide.
-

- $0.00 / 1g
- 2025-01-13
- CAS:364-98-7
- Min. Order: 1g
- Purity: More Than 99%
- Supply Ability: 100kg/Month
- Diazoxide
-

- $50.00 / 100mg
- 2024-11-19
- CAS:364-98-7
- Min. Order:
- Purity: 99%
- Supply Ability: 10g