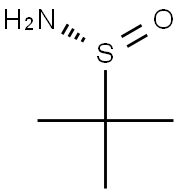Explore (R)-(+)-2-Methyl-2-propanesulfinamide: a key role in chiral chemical synthesis
Jul 2,2024
Abstract
In the vast field of chemistry, chiral molecules have become the focus of research in the fields of synthetic chemistry, medicinal chemistry, and biochemistry due to their unique spatial structure and properties. Among them, (R)-(+)-2-Methyl-2-propanesulfinamide, as an important chiral chemical, has attracted extensive attention in the scientific community for its unique chemical properties and wide application prospects.1
Chemical properties
(R)-(+)-2-Methyl-2-propanesulfinamide, chemical formula C4H11NOS, is a white or white-like solid with a melting point of about 103-107 ° C. As a chiral ligand, it plays an irreplaceable role in organic synthesis. This compound has a unique sulfinamide structure, which makes it have good reactivity and stability. It can react with aldehydes, ketones, and other compounds to form P, n-sulfonamide ligands, and further participate in the asymmetric hydrogenation of olefin catalyzed by iridium. In addition, it also has certain oxidation resistance and thermal stability, which can protect other compounds in the reaction system to a certain extent.2
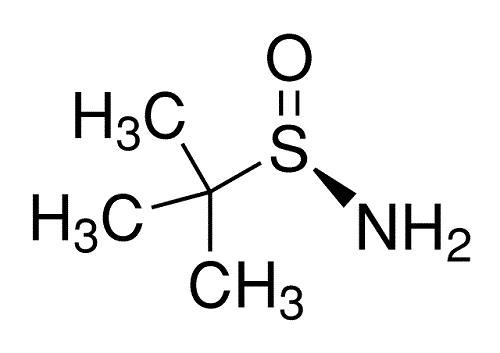
The synthesis method
There are many ways to synthesize (R)-(+)-2-Methyl-2-propanesulfinamide, among which the more common ones are lithium-ammonia reduction and chiral resolution.
Lithium - ammonia reduction method
The lithium-ammonia reduction method is a method to obtain the product from tert-butyl mercaptan through a series of chemical reactions. First, tert-butyl mercaptan oxidizes with hydrogen peroxide under the action of a catalyst to produce tert-butyl thiosulfate. Then, in the presence of liquid ammonia, lithium metal, and ferric nitrate, the tert-butylthiosulfoester is reduced to (R)-(+)-2-Methyl-2-propanesulfinamide. This method has the advantages of easy raw materials, simple operation, and high yield, and is one of the main methods of preparation and production at present.3
Chiral resolution
The chiral resolution method is a method to separate the racemic test-butyl sulfonamide into two chiral configurations of R type and S type by using chiral resolution agents. Firstly, the racemized test-butyl sulfonamide was mixed with a chiral resolver, and the R-type and S-type chiral configurations were separated by crystallization and filtration. Then, the purity and yield of the product were further improved by recrystallization and other methods. This method is suitable for the preparation of high purity (R)-(+)-2-Methyl-2-propanesulfinamide, but the operation process is complicated and the cost is high.4
Application field
(R)-(+)-2-Methyl-2-propanesulfinamide has a wide range of applications in organic synthesis, drug synthesis, and biochemistry.5
Field of organic synthesis
In the field of organic synthesis, (R)-(+)-2-Methyl-2-propanesulfinamide is often used as an intermediate in organic synthesis, participating in the preparation of a variety of complex organic compounds. For example, it can undergo condensation reactions with aldehydes, ketones, and other compounds to generate P, n-sulfonamide ligands, and further participate in reactions such as asymmetric hydrogenation of olefin catalyzed by iridium. In addition, it can also be used as an organic catalyst for β-chlorosulfamide synthesis and enantioselective reduction of imine, which provides strong support for the development of organic synthesis.
Field of drug synthesis
In the field of drug synthesis, (R)-(+)-2-Methyl-2-propanesulfinamide is widely used in the synthesis of chiral amines. Chiral amines are important components of many drugs and have unique biological activities and pharmacological effects. By using it as a chiral chemical, drug molecules with specific chiral structures can be effectively synthesized, thereby improving the efficacy of drugs and reducing side effects.
Field of biochemical
In the field of biochemistry, (R)-(+)-2-Methyl-2-propanesulfinamide also has a certain application value. It can interact with some enzymes in vivo and affect the catalytic activity and stability of enzymes. In addition, it can also be used as a biological probe and molecular marker for the study of some biological processes and mechanisms in organisms.
Future development trend
With the continuous development and innovation of science and technology, the research and application of (R)-(+)-2-Methyl-2-propanesulfinamide will also usher in new development opportunities. In the future, its research will pay more attention to the optimization and improvement of its synthesis methods to improve the purity, yield, and stability of the products. At the same time, people will further explore its new applications and new functions in the fields of organic synthesis, drug synthesis, and biochemistry. In addition, with the continuous improvement of environmental awareness, future research will also pay more attention to its green synthesis and environmental protection applications.
References
[1].Guan, X.-Y., 2-Methyl-2-propanesulfinamide (Ellman’s Sulfinamide): A Versatile Chiral Reagent. Synlett 2010, 2010 (03), 503-504.
[2].Qamar, M. I. Synthesis and Reactions of Cyclopropenones. University of Huddersfield, 2011.
[3].Dallanoce, C.; Matera, C.; Amici, M. D.; Rizzi, L.; Pucci, L.; Gotti, C.; Clementi, F.; Micheli, C. D., The enantiomers of epiboxidine and of two related analogs: Synthesis and estimation of their binding affinity at α4β2 and α7 neuronal nicotinic acetylcholine receptors. Chirality 2012, 24 (7), 543-551.
[4].Mistico, L.; Ay, E.; Huynh, V.; Bourderioux, A.; Chemla, F.; Ferreira, F.; Oble, J.; Perez-Luna, A.; Poli, G.; Prestat, G., Reactivity of tert-butanesulfinamides in palladium-catalyzed allylic substitutions. Journal of Organometallic Chemistry 2014, 760, 124-129.
[5].Grellepois, F.; Jamaa, A. B.; Rosa, N. S., α-Trifluoromethylated tertiary homoallylic amines: diastereoselective synthesis and conversion into β-aminoesters, γ-and δ-aminoalcohols, azetidines and pyrrolidines. Organic & Biomolecular Chemistry 2017, 15 (45), 9696-9709.
- Related articles
- Related Qustion
This paper will deeply analyze the chemical properties, synthesis methods, application scenarios and future development trends of Dess-Martin periodinane.....
Jul 2,2024APISodium acetate trihydrate (NaC2H3O2·3H2O) is a widely used chemical compound in various industrial and laboratory applications.....
Jul 2,2024API(R)-(+)-2-Methyl-2-propanesulfinamide
196929-78-9You may like
(R)-(+)-2-Methyl-2-propanesulfinamide manufacturers
- (R)-(+)-2-Methyl-2-propane sulfinamide
-

- $10.00 / 5g
- 2024-08-07
- CAS:196929-78-9
- Min. Order: 5g
- Purity: 0.98
- Supply Ability: 25kg
- (R)-(+)-2-Methyl-2-propanesulfinamide
-

- $0.00 / 100G
- 2024-06-11
- CAS:196929-78-9
- Min. Order: 10G
- Purity: 0.98
- Supply Ability: 500kg
- (R)-(+)-2-Methyl-2-propanesulfinamide
-

- $50.00 / 1KG
- 2023-07-29
- CAS:196929-78-9
- Min. Order: 1KG
- Purity: 99%
- Supply Ability: 20tons