Exploring Hematin: Definition, Production, Applications, and Considerations in the Chemical and Medical Fields
Apr 12,2024
Introduction
Hematin, a complex and multifaceted compound, stands at the forefront of numerous scientific fields, with its significance spanning medicine, biochemical engineering, and chemical research. As an integral component of hemoglobin and various essential biomolecules, hematin's role extends beyond the basic life-sustaining functions. It finds remarkable applications in treating diseases, where its properties are harnessed to develop innovative therapeutic approaches. Additionally, in the realm of biotechnology, hematin is utilized in assays and experiments, contributing to advancements in biotechnological techniques and understanding. Its contribution to scientific investigations cannot be overstated, as it enables researchers to delve into the molecular workings of life, thereby paving the way for groundbreaking discoveries and applications in health and industry[1].

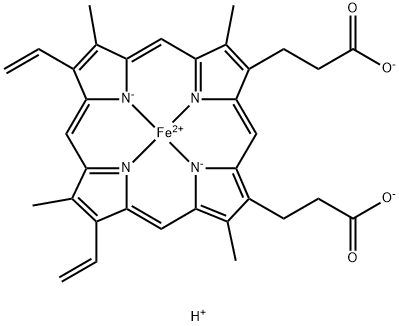
Figure 1 Characteristics of Hematin
Definition and Main Components of Hematin
Hematin is an iron-containing compound, a derivative of heme, which is integral to hemoglobin in blood cells and various other hemoproteins. Structurally, hematin is characterized by an iron atom coordinated in the center of a porphyrin ring, a configuration that is crucial for its biological functions. The iron in hematin can be in the ferric (Fe3+) or ferrous (Fe2+) state, influencing its interaction with oxygen and other molecules.
The main component of hematin is the heme group, which consists of a large, nitrogen-containing organic ring called porphyrin, attached to an iron ion. This structure enables hematin to bind with oxygen, facilitating oxygen transport and storage in living organisms. Moreover, hematin's ability to participate in electron transfer reactions makes it vital in various enzymatic processes.
Production Methods of Hematin
The synthesis of hematin in a laboratory setting involves several sophisticated chemical processes. Typically, hematin is derived from heme, which is extracted from hemoglobin in red blood cells. The conversion of heme to hematin can be achieved through the oxidation of the iron atom within the heme group from the ferrous (Fe2+) to the ferric (Fe3+) state, a process often facilitated by chemical oxidants.
Extraction of Heme
The initial step in producing hematin involves the extraction of heme from sources like red blood cells. This process typically involves the lysis of cells and the separation of heme from other cellular components through various purification techniques.
Chemical Oxidation
Once heme is extracted, it is subjected to oxidation. This chemical reaction involves the addition of an oxidizing agent, which converts the iron ion from its ferrous to ferric state, transforming heme into hematin.
Purification and Characterization
Following oxidation, the resultant hematin is purified through techniques like chromatography or crystallization to remove any impurities. The final product is then characterized using spectroscopic methods to confirm its structure and purity.
Applications of Hematin
Hematin boasts a broad spectrum of applications, significantly influencing various scientific and medical fields due to its unique chemical and biological properties.
Medical Applications
In the healthcare sector, hematin plays a crucial role in treating certain types of porphyria, a group of disorders arising from a buildup of natural chemicals that produce porphyrin in the body. Hematin acts as an enzyme inhibitor, helping reduce the production of porphyrin precursors, thereby alleviating symptoms associated with acute porphyria attacks.
Biochemical Research
Researchers utilize hematin in studying enzymatic processes and in the synthesis of artificial enzymes. Its ability to mimic the behavior of natural enzymes makes it an invaluable tool in biochemical assays and research, aiding in the exploration of enzyme functions and mechanisms.
Industrial Applications
Hematin finds its use in various industrial applications, including the catalysis of certain chemical reactions. Its role as a catalyst in the synthesis of organic compounds is particularly noteworthy, contributing to advancements in chemical manufacturing processes.
Environmental Science
In environmental science, hematin's catalytic properties are leveraged for the breakdown of pollutants. It aids in the development of innovative solutions for waste treatment and environmental remediation, showcasing its versatility beyond biological systems.
Usage Limitations and Contraindications of Hematin
While hematin is invaluable in various applications, its usage is not without limitations and contraindications, particularly in medical contexts where its administration must be carefully monitored[2].
Dosage Limitations
In medical treatments, particularly for acute porphyria, the dosage of hematin must be carefully controlled. Overdosage can lead to complications, including thrombophlebitis (inflammation of the veins) and iron overload, which can be detrimental to the patient's health.
Contraindications
Hematin is contraindicated in patients with a known hypersensitivity to the drug or its components. Furthermore, its use is carefully evaluated in individuals with renal or hepatic impairment, as these conditions can affect the metabolism and excretion of the compound.
Drug Interactions
Hematin can interact with other medications, potentially altering its efficacy or increasing the risk of adverse effects. For instance, simultaneous use of certain anticoagulants may enhance the risk of bleeding, necessitating close monitoring and possible adjustment of dosages.
Storage and Handling
Proper storage and handling of hematin are crucial to maintain its efficacy and safety. It should be stored at controlled room temperatures and protected from light, as improper storage can lead to degradation and loss of effectiveness.
Regulatory Guidelines
Compliance with regulatory guidelines is essential when using hematin in research or medical applications. These guidelines ensure that the compound is used safely and responsibly, minimizing risks to individuals and the environment.
Conclusion
Hematin's multifaceted role across different sectors underscores its importance in scientific and medical fields. From treating rare medical conditions to catalyzing chemical reactions, hematin's applications are vast and varied. However, its usage comes with a set of limitations and contraindications that must be meticulously observed to harness its benefits while mitigating potential risks. As research progresses, the understanding and utility of hematin will continue to evolve, offering promising prospects for future innovations and applications.
References
[1]Glueck R, Green D, Cohen I, et al. Hematin: unique effects of hemostasis[J]. 1983.
[2]Zhang G, Dasgupta P K. Hematin as a peroxidase substitute in hydrogen peroxide determinations[J]. Analytical chemistry, 1992, 64(5): 517-522.
- Related articles
- Hematin: Pharmacodynamics, Clinical Application, Chemical and Toxicity Studies Jan 31, 2023
The Hematin is an iron-containing porphyrin, it consists of an iron (Fe) atom bound to four nitrogen atoms of the pyrrole ring of protoporphyrin IX.
1,7-Dimethylxanthine is a naturally occurring alkaloid compound that can enhance alertness and reduce drowsiness.....
Feb 27,2025API2-Methoxy-4-nitroaniline is an important industrial compound used in dye manufacturing and as an intermediate in the synthesis of other compounds.....
Apr 12,2024APIHematin
14875-96-8You may like
- Spotlight on N-Octyl Pyrrolidone: Antibacterial Activity, Environmental Fate, and Solvent Effects
Apr 17, 2025
- Trimethylolpropane Trimethacrylate: From Industrial Preparation to Mechanical Performance
Apr 17, 2025
- Dihydrocoumarin: An HDAC Inhibitor with Implications for DNA Repair, Aging, and One - Pot Synthesis
Apr 17, 2025
- Hematin
-

- $10.00 / 1kg
- 2025-04-16
- CAS:14875-96-8
- Min. Order: 1kg
- Purity: 99%
- Supply Ability: 100 mt
- Heme Iron
-

- $50.00 / 1KG
- 2025-04-16
- CAS:14875-96-8
- Min. Order: 1KG
- Purity: 2%-8%HPLC,USP Standard
- Supply Ability: 2000KGs
- Hematin
-

- $0.00 / 1kg
- 2025-04-02
- CAS:14875-96-8
- Min. Order: 1kg
- Purity: 99.8%
- Supply Ability: 1000 kg