Hematin: Pharmacodynamics, Clinical Application, Chemical and Toxicity Studies
Jan 31,2023
General Description
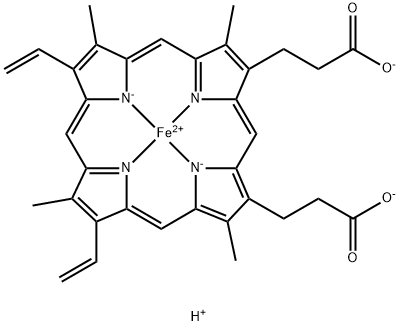
The Hematin, with the CAS No:15489-90-4, as an iron-containing porphyrin, it consists of an iron (Fe) atom bound to four nitrogen atoms of the pyrrole ring of protoporphyrin IX,[17] and can be used to inhibit the activity of clotting factors and also fibrin clotlysis through the mechanism of binding to and inactivation of hemostatic proteins. Its chemical molecular formula is C34H33FeN4O5 and molecular weight is 633.495. Relevant synonyms for hematin include the following names such as heme, haem, haematin and so on. Intravenous (i.v.) hematin has been widely used in the treatment of acute intermittent porph yria (AIP) since the early 1970s and commercially available as Panhematin (hemin for injection; Ovation Pharmaceuticals, Inc.,USA) since 1983, yet no publication to date has attempted to summarize the known pharmacodynamics and toxicological actions of hematin and the implications on treatment.[1] In addition, hematin can be used for the research of the cancer.[1-5] Heme is an essential prosthetic group that forms the reactive core of numerous hemoproteins with diverse biological functions. However, due to its reactive nature, it is also a potentially toxic molecule.[15]
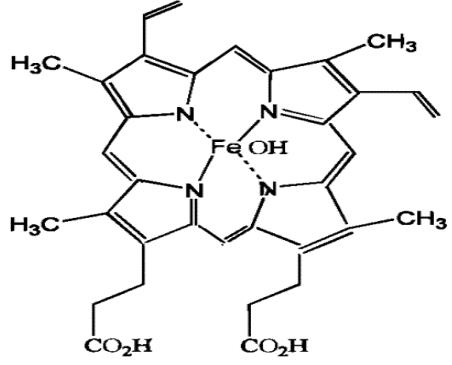
Figure 1 the molecular formula of Hematin
Pharmacodynamics
In Vivo:Hematin (4 mg/kg) was infused intravenously over a I 5-mm period on 3 separate occasions. Blood samples were obtained immediately prior to infusion, and 10 mm, 5 hr, 24 hr. and 48 hr postinfusion.Ten minutes after the infusion, all clotting times were prolonged, the platelet count and levels of factors V. VIII, and fibrinogen had declined, and the titer of FDP had doubled. By 5 hr. there was partial recovery and complete recovery at 48 hr. Platelet aggregation was impaired with ADP and collagen at 10 mm, and although improved at 48 hr. a secondary wave of aggregation was still absent. There were no morphological changes in erythrocytes examined in smears of peripheral blood, and plasma levels of plasminogen and antithrombin III were unaltered.[6]
Clinical Application
Initial reports indicated that hematin was remarkably effective in severe acute porphyric attacks.[7-8] However, the results were not uniform, and a more recent study suggested that hematin was frequently ineffective.[9] There are also reports of side effects, the most disturbing of which describe anticoagulant properties of administered hematin. But, hematin has anticoagulant effects. In the series of Watson and coworkers, six patients treated with freshly prepared hematin responded promptly and completely. Of nine patients at distant facilities who received hematin sent by carrier, only four had convincing evidence of a response. Lamon and colleagues studied 12 patients treated with lyophilized hematin.[10-11] Whereas some of the patients had chronic neurologic symptoms, four had unequivocal acute attacks, and all four responded strikingly to hematin. On the other hand, McColl and coworker concluded that only five of their eight patients with acute attacks had an early symptomatic response to hematin. However, the hematin solutions used in that study were stored at 4℃for as long as 10 days before use.[12]
Preparation
A detailed protocol (BB-IND 1411) is on file with the U.S. Food and Drug Administration. In brief, hemin (ferriprotoporphyrin chloride) was extracted and crystallized from outdated human banked blood.[13] The final product, as the alkaline-pyridine complex, exhibited an E557/541 ratio in the range of 3.35 to 3.45.[14] Hematin for clinical use was prepared by dissolving 1 g of hemin in 100 ml of 0.1M sodium carbonate. The solution was adjusted to a pH of 8.0 with hydrochloric acid and was filter-sterilized and administered within one hour. For the lyophilized preparation, 1 g of sorbitol was added to the hematin solution before sterile filtration. Aliquots of 10 ml were lyophilized and stoppers were inserted under vacuum. The dry material was reconstituted with 10 ml of sterile 0.01 N hydrochloric acid, yielding a solution of pH 8.0.[4] In non-photosynthetic eukaryotes, heme biosynthesis begins with the condensation of succinyl-CoA and glycine, forming δ-aminolevulinic acid (ALA) and subsequent heme biosynthetic pathway involves seven enzymes.[15-16]
Toxicity
The highest i.v. human hematin dose reported in the literature was 12.2 mg/kg (1000 mg) and resulted in acute gastrointestinal pain, paresthesia, and acute tubercular necrosis.[4]
Reference
[1]Siegert S W K, Holt R J. Physicochemical properties, pharmacokinetics, and pharmacodynamics of intravenous hematin: a literature review[J]. Advances in therapy. 2008, 25(9): 842-857.
[2]Green D, Reynolds N, Klein J, et al. The inactivation of hemostatic factors by hematin[J]. The Journal of laboratory and clinical medicine.1983, 102(3): 361-369.
[3]Amin M L, Kim D, Kim S J. Development of hematin conjugated PLGA nanoparticle for selective cancer targeting[J]. European Journal of Pharmaceutical Sciences. 2016, 91: 138-143.
[4]Goetsch C A, Bissell D M. Instability of hematin used in the treatment of acute hepatic porphyria[J]. New England Journal of Medicine. 1986, 315(4): 235-238.
[5]Quadros H C, Silva M C B, Moreira D R M. The role of the iron protoporphyrins heme and hematin in the antimalarial activity of endoperoxide drugs[J]. Pharmaceuticals.2022, 15(1): 60.
[6]Glueck R, Green D, Cohen I, et al. Hematin: Unique effects on hemostasis[J]. Blood.1983, 61(2):243-249.
[7]BANKS A, J Sot. Chem. Ind. (London). 1944, 63, 8.
[8]Lovern J A. Some aspects of recent british studies on antioxidants[J]. Oil Soap.1946, 23(2):40-45.
[9]GEORGE P, AND ROBERTSON A, Trans. Faraday Sot. 1946, 42, 217.
[10]Watson CJ, Pierach CA, Bossenmaier I, Cardinal R. Use of hematin in the acute attack of the inducible hepatic porphyrias[J]. Adv Intern Med. 1978, 23:265-86.
[11]Lamon JM, Frykholm BC, Hess RA, Tschudy DP. Hematin therapy for acute porphyria. Medicine (Baltimore).1979, 58:252-69.
[12]McColl KEL, Moore MR, Thompson GG, Goldberg A. Treatment with haematin in acute hepatic porphyria[J]. Q J Med. 1981, 50:161-74.
[13]Fischer H. Hemin. In: Drake NL, ed. Organic syntheses. London: John Wiley.1941:53-5.
[14]Paul KG, Theorell H, Akeson A. The molar light absorption of pyridine ferroprotoporphyrin[J]. Acta Chem Scand.1953, 7:1284-7.
[15]]Ponka, Prem. Cell biology of heme[J]. American Journal of the Medical Sciences. 1999, 318(4):241-256.
[16]Heinemann IU, Jahn M, Jahn D. The biochemistry of heme biosynthesis[J]. Arch Biochem Biophys. 2008, 474: 238-251.
[17]Kehrer JP. The Haber-Weiss reaction and mechanisms of toxicity[J]. Toxicology. 2000, 149: 43-50.
- Related articles
- Related Qustion
- Exploring Hematin: Definition, Production, Applications, and Considerations in the Chemical and Medical Fields Apr 12, 2024
Hematin plays a pivotal role in various sectors, particularly in medicine, biochemical engineering, and chemical research.
Polycarbosilanes (PCSs) are the essential polymeric precursors to manufacture silicon carbide (SiC) ceramics via a polymer-derived ceramic (PDC) method.....
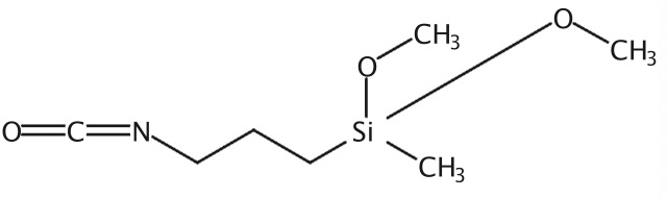
Jan 31,2023APIThe Silane, (3-isocyanatopropyl) dimethoxymethyl, with the CAS No: 26115-72-0, is also known as γ-isocyanatopropylmethyl dimethoxysilane. This chemical’s molecular formula is C7H15NO3Si.....
Feb 1,2023APIHematin
14875-96-8You may like
- Ferroheme
-

- $40.00 / 1mg
- 2025-12-19
- CAS:14875-96-8
- Min. Order:
- Purity: 97.63%
- Supply Ability: 10g
- Hematin
-

- $10.00 / 1kg
- 2025-12-11
- CAS:14875-96-8
- Min. Order: 1kg
- Purity: 99%
- Supply Ability: 100 mt
- Hematin USP/EP/BP
-

- $1.10 / 1g
- 2025-11-18
- CAS:14875-96-8
- Min. Order: 1g
- Purity: 99.9%
- Supply Ability: 100 Tons min