Is acetylene a polar molecule?
Dec 19,2023
Overview
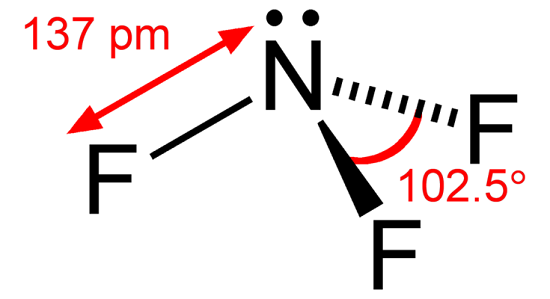
Ethyne, also known as acetylene, is an organic chemical compound with the chemical formula C2H2. Since the entire chemical composition only features hydrogen and carbon atoms, this compound is a hydrocarbon. It is a colorless gaseous chemical compound and the simplest alkyne, i.e., an unsaturated hydrocarbon with a triple C-C Bond. Pure ethyne is known to be highly unstable. Therefore, it is common for ethyne to be handled in a solution. Generally, acetylene is used as a fuel and a chemical building block.
Structure
The ethyne molecule features a triple bond between two carbon atoms, each singly bonded to another hydrogen atom. It can be noted that all four atoms are placed in a single line, with the hydrogen atoms at the corners and carbon atoms with a triple bond between them in the center. In addition, the bond angle is 180°. It can also be noted that the bond length of the carbon-hydrogen bond in the ethyne molecule is roughly equal to 106 picometres, whereas the carbon-carbon bond length in the molecule is approximately equal to 120.3 picometres.

C2H2 is nonpolar in nature. The polarity of any molecule depends on the following factors: The difference in electronegativities of atoms. The shape of the molecule. Dipole moment. Distribution of charges
The electronegativity difference between carbon and hydrogen is 0.35, less than the minimum required 0.4. Moreover, as the difference in electronegativities is not that high, this molecule will have nearly no to zero dipole moment. Furthermore, it has a linear molecular structure, and the nature of C-H bonds is nonpolar covalent. This makes the complete C2H2 molecule a nonpolar molecule with a net zero dipole moment. Moreover, as there is no net dipole moment, there are no poles in this molecule. The charges in the molecule are evenly distributed, and there are no positively charged or negatively charged areas in the molecule. This even distribution of the charges also makes this molecule a nonpolar molecule.
- Related articles
- Related Qustion
- Is acetylene dissolved in water more stable than gaseous acetylene? Mar 6, 2024
When acetylene is dissolved in a liquid, its molecules are separated by solvent molecules, and it is stable and safe to handle at high temperature and pressure as long as it remains in the liquid phase.
- What is the Lewis structure for acetylene? Nov 22, 2023
Acetylene is the simplest and best-known member of the hydrocarbon series containing one or more pairs of carbon atoms linked by triple bonds.
- Acetylene-Health Hazard and Toxicity Sep 2, 2019
Acetylene (100% purity) is odourless, but commercial purity has a distinctive garlic-like odour and is very soluble in alcohol and almost miscible with ethane. Acetylene is a flammable gas and kept under pressure in gas cylinders. Under cer
Supplementation with sodium acetate (NaAcet) increases milk fat production through an apparent stimulation of de novo lipogenesis in the mammary gland.....
Dec 19,2023APINitrogen trifluoride is a chemical compound with chemical formula NF3. It exists as a colorless gas having a musty smell at room temperature.....
Dec 19,2023Inorganic chemistry