L(+)-2-Aminobutyric acid: Innovations in Dental Care and Biocatalytic Synthesis
Jul 17,2024
General Description
L(+)-2-Aminobutyric acid is utilized in dentistry for caries removal through the innovative Caridex system. This system softens carious lesions, easing their removal without extensive drilling, reducing pain and anxiety for patients. While slower than traditional methods, it offers a less invasive alternative. The biosynthesis of L(+)-2-Aminobutyric acid using biocatalytic methods has shown high yields with optimized conditions, providing potential for industrial-scale production. These advances highlight the compound's versatility in medical and dental applications, paving the way for future developments in patient-friendly dental technologies and amino acid synthesis.

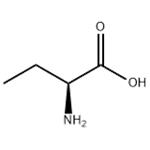
Figure 1. L(+)-2-Aminobutyric acid
Applications in Dentistry
Utilization in Caries Removal
L(+)-2-Aminobutyric acid finds a unique application in dentistry, particularly in the chemomechanical removal of dental caries through the Caridex system. Introduced in the 1970s, the Caridex system utilizes a derivative of L(+)-2-Aminobutyric acid, namely N-monochloro-DL-2 aminobutyrate (NMAB), which is specifically designed for this purpose. This compound is created in situ by mixing two components: a dilute solution of DL-2-aminobutyric acid and sodium hypochlorite in a weakly alkaline solution. The NMAB solution effectively softens carious lesions, which facilitates their removal without the need for extensive mechanical drilling. This innovative application highlights the versatility of L(+)-2-Aminobutyric acid derivatives in medical and dental treatments, providing a less invasive alternative to traditional mechanical caries removal techniques.
Advantages and Mechanism of the Caridex System
The Caridex system, leveraging the properties of L(+)-2-Aminobutyric acid, primarily benefits patients by reducing pain and anxiety associated with dental procedures. The system includes a specialized delivery setup consisting of a pump, heater, solution reservoir, and an applicator handpiece. The NMAB solution, when applied to decayed tooth areas, softens the decayed material, thus allowing for easier removal using conventional dental tools. This method is particularly advantageous for patients who are anxious about dental drills, as it minimizes the need for their use and can make dental care more accessible to those with dental phobias.
Limitations and Clinical Implications
Despite its benefits, the application of L(+)-2-Aminobutyric acid in the Caridex system does have limitations. The process of caries removal using the Caridex system is generally slower than traditional methods, which may not make it suitable for all clinical situations. Dentists must weigh the benefits of a less invasive and less painful procedure against the longer treatment time required. However, the safety and clinical acceptability of L(+)-2-Aminobutyric acid and its derivatives have been well-documented since the system's introduction, with no adverse side effects reported. This positions L(+)-2-Aminobutyric acid as a valuable component in the ongoing development of patient-friendly dental technologies, suggesting potential for broader applications in future dental therapies. 1
Biosynthesis
L(+)-2-Aminobutyric acid, a significant amino acid derivative, plays a crucial role in various biochemical applications. The synthesis of L(+)-2-Aminobutyric acid can be efficiently executed through biocatalytic methods involving recombinant strains of Escherichia coli. This study utilizes two recombinant E. coli strains, both expressing leucine dehydrogenase (LDH) from Bacillus cereus, but paired with different dehydrogenases: formate dehydrogenase (FDH) from Ancylobacter aquaticus and alcohol dehydrogenase (ADH) from Rhodococcus. These engineered strains facilitate the synthesis of L(+)-2-Aminobutyric acid by initiating the deamination of L-threonine, a precursor amino acid. The incorporation of an NADH regeneration system is pivotal, ensuring the sustainability and efficiency of the reaction by maintaining the necessary co-factor balance.
Optimization of Synthesis Processes
For the synthesis of L(+)-2-Aminobutyric acid, the optimization of reaction conditions is paramount. The study details the optimization of two processes, namely LDH-FDH and LDH-ADH systems. The LDH-FDH system was found optimal at a pH of 7.5 and a temperature of 35°C. Under these conditions, after 28 hours, L(+)-2-Aminobutyric acid was produced at a concentration of 161.8 g/L, achieving a yield of 97%. This was facilitated by batch addition of L-threonine to control the concentration of 2-ketobutyric acid and using 50 g/L ammonium formate alongside 0.3 g/L NAD+. In contrast, the LDH-ADH system, optimal at a pH of 8.0, yielded 119.6 g/L of L(+)-2-Aminobutyric acid at a similar temperature and time frame, with the innovative step of isopropanol addition enhancing yield to 98%.
Implications for Industrial Application
The results of this study underscore the potential for industrial-scale production of L(+)-2-Aminobutyric acid utilizing biotechnological approaches. Both systems demonstrated high yields under optimized conditions, making them viable for commercial applications. Moreover, the use of biocatalysis for the synthesis of L(+)-2-Aminobutyric acid not only improves efficiency but also enhances the environmental friendliness of the production process. These findings provide a solid foundation for further research and development in the field of amino acid synthesis, particularly for compounds that are critical in medicine and industry. The study’s methodologies and results could serve as a reference point for scaling up the production of L(+)-2-Aminobutyric acid and potentially other valuable amino acids. 2
Reference
1. Vougiouklakis G, Paroussis D. Chemikomechanike aphairese teredonas (Systema CARIDEX) [Chemomechanical means of removing caries--Caridex system]. Hell Stomatol Chron. 1988; 32(2): 97-102.
2. Zhang LK, Xiao YM, Yang WH, et al. Synthesis of L-2-aminobutyric acid by leucine dehydrogenase coupling with an NADH regeneration system. Chinese Journal of Biotechnology, 2020, 36(5): 992-1001.
- Related articles
- Related Qustion
L-Tyrosine is the left-handed isomer of the aromatic amino acid tyrosine. It is a naturally occurring tyrosine that is synthesised in the body from L-phenylalanine.....
Jul 16,2024Amino Acids and ProteinsCalcium gluconate, a compound widely used in the medical and food industries, plays an essential role in various biological processes.....
Jul 17,2024APIL(+)-2-Aminobutyric acid
1492-24-6You may like
L(+)-2-Aminobutyric acid manufacturers
- L-2-Abu
-
- $0.00/ kg
- 2024-08-27
- CAS:1492-24-6
- Min. Order: 1kg
- Purity: 99%
- Supply Ability: 1T+
- L(+)-2-Aminobutyric acid
-

- $35.00 / 1KG
- 2024-08-27
- CAS:1492-24-6
- Min. Order: 1KG
- Purity: 0.99
- Supply Ability: 20 tons
- L(+)-2-Aminobutyric acid
-

- $99.00/ kg
- 2024-08-27
- CAS:1492-24-6
- Min. Order: 0.00100000kg
- Purity: 99%
- Supply Ability: 5000





