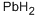Lead-Hazard and Toxicity
Sep 9,2019
Description
Lead is a bluish-white, heavy metallic element with properties that are more metal-like than the properties of metal loids or nonmetals. Lead can be found in its native state, meaning that elemental metallic lead can be found in deposits in the Earth’s crust. However, most lead is first mined as galena ore (lead sulfide, PbS). The galena is mixed with lead sulfate, lead sulfide,and lead oxide and is then roasted at a high temperature. The air supply is reduced, followed by an increase in heat and the vaporization of the sulfates and oxides of lead, which are drawn off as gases. The molten lead is then recovered.

Lead is only slightly soluble in water. However, it is also toxic. This is the reason lead is no longer used to pipe fresh water into homes. It does not react well with acids, with the exception of nitric acid. Lead’s melting point is 327.46°C, its boiling point is 1,740°C, and its density is 11.342 g/cm3.
Toxicity Data
LDLO oral (pigeon) 160 mg/kg
PEL (OSHA) 0.05 mg/m3
PEL (action level) 0.03 mg/m3
TLV-TWA (ACGIH) 0.05 mg/m3
(PEL and TLV apply to lead and inorganic lead compounds)
Major Hazards
Chronic toxin affecting the kidneys and central and peripheral nervous systems; reproductive and developmental toxin. Lead is probably one of the most widely distributed poisons in the world. Not only is the metal poisonous, but most lead compounds are also extremely toxic when inhaled or ingested.A few, such as lead alkalis, are toxic when absorbed through skin contact.
Workers in industries using lead are subject to testing of their blood and urine to determine the levels of lead in their bodies’ organs. Great effort is made to keep the workers safe.Unfortunately, many older homes (built prior to 1950) have several coats of lead-based paints that flake off, which then may be ingested by children, causing various degrees of lead poisoning, including mental retardation or even death.
Another hazardous source of lead is pottery that is coated with a lead glaze that is not stabilized. Acidic and hot liquids (citrus fruits, tea, and coffee) react with the lead, and each use adds a small amount of ingested lead that can be accumulative. Lead air pollution is still a problem, but not as great as before, given that tetra ethyl lead is no longer used in gasoline.However, lead air pollution remains a problem for those living near lead smelting operations or in countries where leaded gasoline is still permitted.
Even though lead and many of its compounds are toxic and carcinogenic, our lives would be much less satisfying without its use in our civilization.
Toxicity
The acute toxicity of lead and inorganic lead compounds is moderate to low. Symptoms of exposure include decreased appetite, insomnia, headache, muscle and joint pain, colic, and constipation. Inorganic lead compounds are not significantly absorbed through the skin.
Chronic exposure to inorganic lead via inhalation or ingestion can result in damage to the peripheral and central nervous system, anemia, and chronic kidney disease. Lead can accumulate in the soft tissues and bones, with the highest accumulation in the liver and kidneys, and elimination is slow. Lead has shown developmental and reproductive toxicity in both male and female animals and humans. Lead is listed by IARC in Group 2B ("possible human carcinogen") and by NTP as "reasonably anticipated to be a carcinogen," but is not considered to be a "select carcinogen" under the criteria of the OSHA Laboratory Standard.
Flammability and Explosibility
Lead powder is combustible when exposed to heat or flame.
Reactivity and Incompatibility
Violent reactions of lead with sodium azide, zirconium, sodium acetylide, and chlorine trifluoride have been reported. Reactivity of lead compounds varies depending on structure.
Storage and Handling
In particular, work with lead dust, molten lead, and lead salts capable of forming dusts should be conducted in a fume hood to prevent exposure by inhalation.
Accidents
In the event of skin contact, immediately wash with soap and water and remove contaminated clothing. In case of eye contact, promptly wash with copious amounts of water for 15 min (lifting upper and lower lids occasionally) and obtain medical attention. If lead or lead compounds are ingested, obtain medical attention immediately.
If large amounts of such substances are inhaled, move the person to fresh air and seek medical attention at once. In the event of a spill, sweep up dry lead and its compounds, soak up solutions with a spill pillow or absorbent material, place in an appropriate container, and dispose of properly. Respiratory protection may be necessary in the event of a large spill or release causing significant airborne particulate levels.
Disposal
Excess lead and waste material containing this substance should be placed in an appropriate container, clearly labeled, and handled according to your institution's waste disposal guidelines.
- Related articles
- Related Qustion
- How are Lead Minerals Distributed? May 31, 2024
Many lead minerals are relatively light and, in Earth’s history, have stayed in the crust instead of sinking deeper into the Earth’s interior.
Iodine is widely distributed in nature and it exists in the form of compounds. There are traces of iodine in rocks, soil, water, flora and fauna and air. But in addition to seawater, the distribution of iodine in nature is very uneven. The....
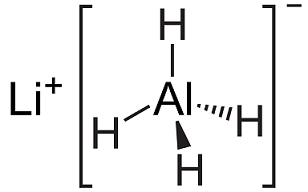
Sep 9,2019Inorganic chemistryLithium aluminium hydride, commonly abbreviated to LAH, is an inorganic compound with the chemical formula LiAlH4. It was discovered by Finholt, Bond and Schlesinger in 1947. This compound is used as a reducing agent in organic synthesis, e....
Sep 9,2019Inorganic chemistryLead
7439-92-1You may like
- Lead Powder
-

- $63.00/ kg
- 2024-10-29
- CAS:7439-92-1
- Min. Order: 1000kg
- Purity: 98%
- Supply Ability: 10T
- Lead
-

- $10.00 / 1KG
- 2021-10-19
- CAS:7439-92-1
- Min. Order: 1KG
- Purity: 99%
- Supply Ability: 20 tons
- Lead
-

- $1.00 / 1KG
- 2019-09-02
- CAS:7439-92-1
- Min. Order: 1KG
- Purity: 85.0-99.8%
- Supply Ability: 200kg