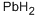How are Lead Minerals Distributed?
May 31,2024
Lead is classified as a chalcophile under the Goldschmidt classification (developed by Victor Goldschmidt (1888-1947), a geochemical classification which groups the chemical elements within the Earth according to their preferred host phases into lithophile (rock-loving), siderophile (iron-loving), chalcophile (ore-loving or chalcogen-loving), and atmophile (gasloving) or volatile (the element, or a compound in which it occurs, is liquid or gaseous at ambient surface conditions)), meaning it is normally found in combination with sulfur. It rarely occurs as its native metal.

Distribution of lead minerals
Many lead minerals are relatively light and, in Earth’s history, have stayed in the crust instead of sinking deeper into the Earth’s interior. This explains the relatively high crustal abundance of 14 ppm; it is the 38th most abundant element in the crust. The most common leadbearing mineral is galena (PbS), which is typically found together with zinc ores (mainly as sphalerite ZnS). Most other lead minerals are related to galena in some way; anglesite, PbSO4, is a product of galena oxidation; and cerussite or white lead ore, PbCO3, is a decomposition product of galena. Sb, Sn, As, Ag, Au, Cu, and Bi are common impurities in Pb minerals. Global Pb resources surpass 2 billion tons. Significant deposits are found in Australia, China, Ireland, Mexico, Peru, Portugal, Russia, and the United States. Worldwide reserves are estimated to total 88 million tons.
Lead-related compounds
Pb also reacts with the other members of group 16. The heavier members of group 16 (S, Se, and Te referred to as chalcogens) react with molten Pb give the chalcogenides PbS, PbSe and PbTe. Pure PbS can be made by direct reaction of the elements or by reaction of Pb(OAc)2 with thiourea. Reaction of lead with sulfur or hydrogen sulfide produces lead sulfide. The solid has the NaCl-like structure (simple cubic) up to its melting point at 1114℃. If the heating occurs in presence of air, it decomposes to form the monoxide and the sulfate. The compound is nearly insoluble in water, weak 824 The Periodic Table: Nature’s Building Blocksacids, and (NH4)2S/(NH4)2S2 solution is the key for separation of lead from analytical groups I to III elements, tin, arsenic, and antimony. The compound dissolves in nitric and hydrochloric acids, to form elemental sulfur and hydrogen sulfide, respectively. Heating mixtures of the monoxide and the sulfide produces the metal.
- Related articles
- Related Qustion
- Lead-Hazard and Toxicity Sep 9, 2019
Lead is a bluish-white, heavy metallic element with properties that are more metal-like than the properties of metal loids or nonmetals. Lead can be found in its native state, meaning that elemental metallic lead can be found in deposits in
Supplementation with pyridoxal 5'-phosphate monohydrate can synthesize neurotransmitters such as dopamine and serotonin, maintaining a healthy nervous system.....
Nov 4,2025Biochemical EngineeringPlatinum is a very rare metal, occurring at a concentration of only around 0.005 ppm in the Earth’s crust. Pt is often found as the native element and as alloys with the other Pt-group elements and Fe mostly.....
May 31,2024APILead
7439-92-1You may like
- Lead Powder
-

- $63.00/ kg
- 2024-10-29
- CAS:7439-92-1
- Min. Order: 1000kg
- Purity: 98%
- Supply Ability: 10T
- Lead
-

- $10.00 / 1KG
- 2021-10-19
- CAS:7439-92-1
- Min. Order: 1KG
- Purity: 99%
- Supply Ability: 20 tons
- Lead
-

- $1.00 / 1KG
- 2019-09-02
- CAS:7439-92-1
- Min. Order: 1KG
- Purity: 85.0-99.8%
- Supply Ability: 200kg