Acephate: toxic effects, physicochemical and microbial degradation
Jan 19,2024
General Description
Polyglutamic acid is challenging to synthesize through ribosomal synthesis due to codon similarities and limited availability of ribosomal subunits. However, alternative approaches are being explored. In the food industry, polyglutamic acid and its derivatives enhance taste, delay aging, and preserve food. It can also increase calcium concentration in health food and improve stability in skincare products. In drug delivery, polyglutamic acid nanoparticles have applications in oral administration, targeting cancer cells, protein entrapment, and antioxidant effects. These nanoparticles can be adjusted in size and surface properties. Overall, polyglutamic acid has diverse applications and shows promise in various industries due to its unique properties and potential benefits.

Figure 1. Polyglutamic acid
Microbiological Synthesis
Microbiological synthesis of polyglutamic acid has proven to be a challenging task despite the initial idea of using ribosomal synthesis with an artificial gene as a template. Previous experiments have reported the production of a short polypeptide similar to polyglutamic acid in a recombinant Escherichia coli strain. Additionally, another experiment involving a fusion protein of dihydrofolate reductase with a C-terminal section consisting of glutamic acid showed that the accumulation of the fusion protein was inversely related to the level of induction of its mRNA. The difficulty in synthesizing monodisperse polyglutamic acid through ribosomal synthesis can be attributed to the similarity between the codons coding for glutamic acid and the Shine-Delgarno sequence. The Shine-Delgarno sequence is crucial for the initiation of translation by ribosomes, as it complements the 3' end of 16S rRNA. Consequently, when a large number of small ribosomal subunits bind to the coding sequence of the artificial polyglutamic acid gene, there is a limited availability of free subunits for protein synthesis initiation. These findings suggest that alternative approaches may be required to achieve efficient synthesis of monodisperse polyglutamic acid. Further research is needed to overcome these challenges and develop reliable methods for microbiological synthesis of polyglutamic acid. 1
Applications in the Food and Drug Delivery Industries
Food Industry
Polyglutamic acid and its derivatives have a wide range of applications in the food industry. In juice and other drinks, low concentrations of Polyglutamic acid have been shown to improve taste and drinkability. In wheat-flour-based foods such as bread, cakes, and pasta, the addition of Polyglutamic acid or its salts can delay aging, improve texture, and retain shape. The antifreeze properties of various Polyglutamic acid salts make them useful in preserving food, microorganisms, and enzymes. The salts have little taste and can be used in greater concentrations than previously used antifreeze agents. Additionally, the calcium salt of Polyglutamic acid can be added to health food to increase Ca2+ concentration, contributing to the prevention of osteoporosis. In skin care products containing water-soluble and insoluble vitamins, the addition of Polyglutamic acid can promote vitamin absorption, increase stability, and provide a sustained-release effect without causing irritation to the skin. Overall, Polyglutamic acid and its derivatives have diverse applications in various industries, including food and skincare, due to their unique properties and potential health benefits. 2
Drug Delivery Industry
Polyglutamic Acid nanoparticles have various applications in drug delivery, including the oral administration of hydrophobic drugs and proteins, targeting liver cancer cells, and entrapping proteins. The nanoparticles are typically formed through an ionic mechanism or emulsion/solvent evaporation technique, and their size and ζ-potential can be adjusted by altering the reaction mixture composition. For oral drug delivery, Polyglutamic Acid nanoparticles formed with chitosan can penetrate Caco-2 cells, which mimic small intestine cells. On the other hand, nanoparticles designed to target liver cancer have galactosamine conjugated to their surface and selectively target cells expressing the liver-specific ASGP receptor. Additionally, Polyglutamic Acid nanoparticles modified with L-phenylalanine esters can be stable in water for up to ten days and show no cytotoxicity. Protein entrapment in Polyglutamic Acid nanoparticles is also achievable by forming a CaCO3 core and multiple outer layers of PEG with Polyglutamic Acid and PEG with polylysine. Alternatively, nanoparticles of Polyglutamic Acid-fullerene conjugates can remove the superoxide radical efficiently. Overall, Polyglutamic Acid nanoparticles have potential applications in drug delivery due to their adjustable properties and biocompatibility. 3
Reference
1. Zhang G, Fournier MJ, Mason TL, and Tirrell DA. Biological synthesis of monodisperse derivatives of poly(alpha,L-glutamic acid): model rodlike polymers. Macromolecules, 1992, 25: 3601–3603.
2. Buescher JM, Margaritis A. Microbial biosynthesis of polyglutamic acid biopolymer and applications in the biopharmaceutical, biomedical and food industries. Crit Rev Biotechnol, 2007, 27(1): 1-19.
3. Sung HW, Liang HF, and Tu H. Nanoparticles for paracellular drug delivery. 2006, United States Patent: 20060073210.
General Description
The extensive and prolonged use of acephate has been found to have toxic effects on a wide range of organisms, including aquatic and terrestrial animals, as well as humans. Studies on model organisms such as zebrafish, fruit flies, and earthworms have revealed developmental delays, deformities, DNA damage, impaired immune response, and other physiological effects due to exposure to acephate. It has also been identified as a genotoxin, causing chromosomal changes and DNA damage in human lymphocytes and affecting human sperm. Physicochemical degradation methods, including oxidation, ultrasonic degradation, ozonization, and chemical degradation, show promise in breaking down acephate into less toxic compounds. Additionally, various microbial strains, such as Pseudomonas sp. and Mucor sp., have shown potential in degrading acephate. Further research and field testing are needed in environmental remediation.

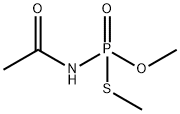
Figure 1. Acephate
Toxic Effects
The extensive and prolonged use of acephate has been found to have toxic effects on a wide range of organisms, including aquatic and terrestrial animals, as well as humans. These pesticides, when released into the environment, can directly or indirectly poison non-target organisms, causing significant harm. Studies conducted on zebrafish, a commonly used model organism, have revealed that exposure to methamidophos can affect neurodevelopmental genes and lead to early neurodamage during development. Exposure to acephate has been shown to cause developmental delays, deformities, and reduced surface tension in zebrafish embryos. Additionally, residues of acephate in water can accumulate and be ingested by aquatic organisms, resulting in adverse effects on the food chain. Other model organisms such as fruit flies and earthworms have also exhibited toxic effects when exposed to acephate. Furthermore, acephate have been found to have detrimental effects on various physiological processes in animals, including alterations in glucose metabolism, reproductive barriers, DNA damage, and impaired immune response. In terms of human health, acephate has been identified as a genotoxin, capable of causing chromosomal changes and DNA damage in human lymphocytes. It has also been shown to have cytotoxic and genotoxic effects on human sperm, affecting motility, cell membrane integrity, and volume. The accumulation of acephate residues in the environment has raised concerns about their impact on various biological systems and health issues. 1
Physicochemical Degradation
Physicochemical degradation methods play a crucial role in the degradation of acephate, an organophosphorus pesticide. Various effective methods have been studied to break down acephate into less toxic compounds. One such method involves the use of colloidal manganese dioxide, which acts as an oxidizing agent in the absence of surfactants, effectively degrading acephate. Another physical method is ultrasonic degradation, which involves mechanical bond breaking and free radical reactions. High-intensity ultrasound generates negative pressure, high temperature, and a high-pressure environment, leading to the formation of potent oxidants like hydrogen peroxide radicals and hydroxyl radicals. These radicals actively contribute to the degradation of acephate. Combining ultrasound with ozonization has shown synergistic effects in degrading acephate. Ultrasound irradiation enhances the oxidizability of nitrogen atoms, improving the efficiency of acephate ozonization. Chemical degradation of acephate involves the destruction of various bonds and the generation of intermediate products. Different free radicals, such as e−aq, negative hydrogen ions (•H), and hydroxyl radicals (•OH), play distinct roles in the degradation process. The reaction kinetics of these radicals lead to two different degradation pathways of acephate, ultimately resulting in the formation of inorganic salts as final products. In summary, physicochemical degradation methods, including oxidation, ultrasonic degradation, ozonization, photocatalysis, and chemical degradation, show significant promise in breaking down acephate into less toxic compounds. 2
Microbial Degradation
There are various microbial strains capable of degrading acephate. The degradation mechanisms involve two main pathways: methamidophos or O-methyl-N-acetylphosphoramidate. Some notable microbial strains include Pseudomonas sp. Ind01, Pseudomonas pseudoalcaligenes PS-5, Pseudomonas aeruginosa Is-6, and a combination of multiple strains including Bacillus cereus ADI-10, Nibacillus fusiformis ADI-01, Pseudomonas pseudoalcaligenes ADI-03, Pseudomonas sp. ADI-04, and Pseudomonas pseudoalcaligenes ADI-06. Microbial fixation can improve microbial utilization and degradation efficiency. Recombinant Escherichia coli containing organophosphorus hydrolase (OPH) encoding plasmid was immobilized in polyvinyl alcohol (PVA) cryogel to form a biocatalyst with high activity, stability, and mechanical strength. Fungi such as Mucor sp. can also be potential degrading microorganisms due to their extensive mycelium networks and low specificity of degrading enzymes. Overall, these microbial strains have shown promising potential in environmental remediation, but more field testing is needed. 3
Reference
1. Lin Z, Pang S, Zhang W, Mishra S, Bhatt P, Chen S. Degradation of Acephate and Its Intermediate Methamidophos: Mechanisms and Biochemical Pathways. Front Microbiol. 2020, 11: 2045.
2. Qamruzzaman, and Nasar A. Degradation of acephate by colloidal manganese dioxide in the absence and presence of surfactants. Desalin. Water Treat. 2014. 8: 2155–2164.
3. Pinjari AB, Novikov B, Rezenom YH, Russell DH, Wales ME, and Siddavattam D. Mineralization of acephate, a recalcitrant organophosphate insecticide is initiated by a Pseudomonad in environmental samples. PLoS One. 2012, 7: e31963.
- Related articles
- Related Qustion
- Acephate: toxic effects, physicochemical and microbial degradation Jan 19, 2024
Acephate has toxic effects on organisms, causing DNA damage, and impaired immune response. physicochemical and microbial degradation show promise in reducing its toxicity.
- A widely used pesticide: Acephate Oct 23, 2023
Acephate is a low-toxicity organophosphate insecticide. But it is easily absorbed by plants and accumulated in edible parts of plants.
4,4'-Methylenedianiline(MDA) is primarily used to produce 4,4-'methylenedianiline diisocyanate and other polymeric isocyanates which are used to manufacture polyurethane foams.....
Dec 10,2024Organic ChemistryAcephate has toxic effects on organisms, causing DNA damage, and impaired immune response. physicochemical and microbial degradation show promise in reducing its toxicity.....
Jan 19,2024API4,4'-Methylenedianiline(MDA) is primarily used to produce 4,4-'methylenedianiline diisocyanate and other polymeric isocyanates which are used to manufacture polyurethane foams.....
Dec 10,2024Organic ChemistryAcephate has toxic effects on organisms, causing DNA damage, and impaired immune response. physicochemical and microbial degradation show promise in reducing its toxicity.....
Jan 19,2024APIAcephate
30560-19-1You may like
- Acephate
-

- $0.00 / 1KG
- 2025-04-15
- CAS:30560-19-1
- Min. Order: 1KG
- Purity: 99%
- Supply Ability: 500000kg
- Acephate
-

- $0.00 / 25kg
- 2025-04-11
- CAS:30560-19-1
- Min. Order: 100kg
- Purity: 98
- Supply Ability: 10000
- Acephate
-

- $100.00 / 25kg
- 2025-03-21
- CAS:30560-19-1
- Min. Order: 1kg
- Purity: 99%
- Supply Ability: 50000KG/month