Pharmacodynamics and Toxicity of Terconazole
Mar 30,2022
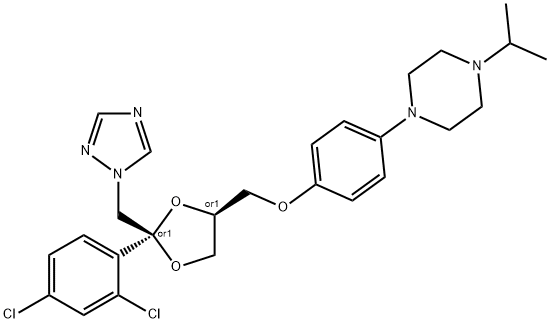
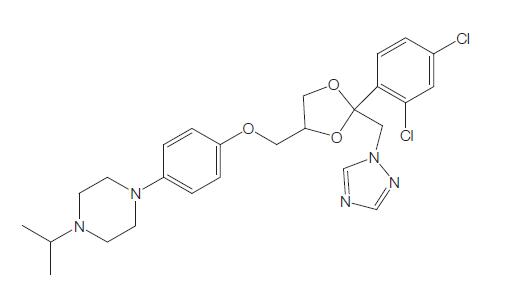
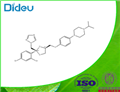
Terconazole is a novel triazole ketal that was synthesized and developed by Janssen Pharmaceutica in 1983. It was the first triazole antifungal agent marketed. It has the chemical name cis-1-{p- [[2-(2,4-dichlorophenyl)-2-(1H-1,2,4-triazol-1-ylmethyl)-1,3-dioxalan- 4-yl]methoxy]phenyl}-4-isopropylpiperazine; the chemical formula is C26H31Cl2N5O3 and the molecular structure is shown below. It has a broad spectrum of activity against dermatophytes, pathogenic yeast, and Candida spp. It is marketed for topical use only.
Mechanism of action
Terconazole binds to the heme iron component of the fungal cytochrome P450 enzyme lanosterol C-14a-demethylase, inhibiting the conversion of lanosterol to ergosterol. The resultant membrane depletion of ergosterol causes an increase in membrane permeability and altered membrane enzyme activity. Terconazole has a significantly greater affinity for the fungal cytochrome P450 enzyme compared with mammalian cytochrome P450. Because of this, there is a decreased tendency for the drug to induce hepatic cytochrome P450 enzyme activity, and consequent induction of metabolism of the drug in vaginal tissue or epithelium. Terconazole also causes a significant increase in chitin in C. albicans cell walls, which may reflect the antifungal activity of the drug.
Pharmacokinetics and Pharmacodynamics
Approximately 5–16% of terconazole administered intravaginally is absorbed into the systemic circulation. In normal healthy volunteers, mean peak serum terconazole levels of 0.01 mg/ml were measured at 7 hours after administration. Peak levels are similar after multiple dosing, and in women with vaginitis. Systemically absorbed drug is rapidly metabolized by the liver and excreted in the urine and feces (Product Information Terconazole, 1994).
Dosage
Terconazole is available in a number of topical formulations, including 80-mg vaginal pessaries, and vaginal creams of 0.4% and 0.8% terconazole. For vulvovaginal candidiasis the recommended dose is one 80-mg vaginal pessary or 5 g of 0.8% vaginal cream inserted once daily for three consecutive nights, or, alternatively, a 5-g dose (1 applicator) of 0.4% vaginal cream administered intravaginally for seven consecutive nights.
Toxicity
Local reactions such as irritation, itching, and burning are reported after vaginal administration of terconazole in women. Skin rash has also been described rarely. A flu-like syndrome has been described in patients who received 160 mg as a single intravaginal dose. Chills and fever begin about 1 hour after administration and are associated with headache and hypotension. Symptoms resolve in 10–48 hours. Over 100 cases have been reported, and it has been hypothesized the symptoms may be a Herxheimer reaction. Approximately 30% of women complain of headache which subsides spontaneously. It occurs after administration of both the vaginal cream and the suppository, and may be a mild manifestation of the flu-like reaction.
- Related articles
- Related Qustion
- Mechanism of Action of Terconazole Feb 28, 2025
The trypanocidal activity of terconazole was evaluated in different stages of the T. cruzi life cycle using parasites from two discrete typing units (DTU) and the mechanism of action was predicted by molecular docking.
- What is a terconazole used for? Mar 16, 2024
The triazole antimycotic terconazole was first synthesized at Janssen Pharmaceuticals. Terconazole is a broad-spectrum triazole antimycotic with activity against most species of pathogenic fungi.
Sulconazole has a broad spectrum of antifungal activity in vitro and has been shown to be an effective topical antifungal agent for the management of superficial fungal infections of the skin, particularly dermatophytoses and tinea versicol....
Mar 30,2022APItioconazole inhibits sterol biosynthesis by the inhibition of cytochrome P450-dependent C-14a-demethylation of lanosterol, preventing conversion to ergosterol, which results in depletion of normal fungal sterols (ergosterol) and accumulatio....
Mar 30,2022APITerconazole
67915-31-5You may like
- Terconazole
-

- $700.00/ g
- 2025-12-16
- CAS:67915-31-5
- Min. Order: 0.0010000000474974513g
- Purity: 99%
- Supply Ability: 5000
- Terconazole
-

- $0.00 / 1kg
- 2025-12-16
- CAS:67915-31-5
- Min. Order: 1kg
- Purity: 98%
- Supply Ability: Customise
- Terconazole USP/EP/BP
-
- $1.10 / 1g
- 2025-11-18
- CAS:67915-31-5
- Min. Order: 1g
- Purity: 99.9%
- Supply Ability: 100 Tons min