Pharmacodynamics and Toxicity of Tioconazole
Mar 30,2022
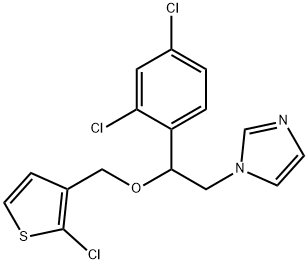
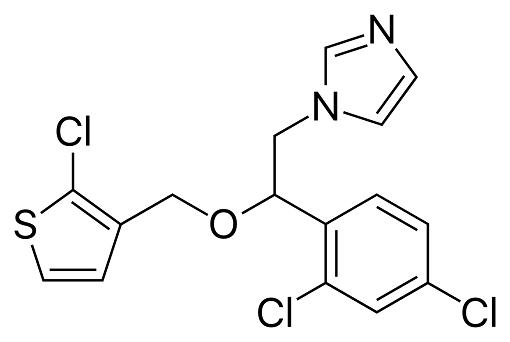
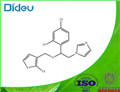
Tioconazole is a substituted imidazole derivative, structurally related to econazole, clotrimazole, and miconazole, with the chemical name 1-[2,4-dichloro-b-[(2-chloro-3-thenyl)oxy]-phenethyl]imidazole; the chemical formula is C16H13Cl3N2OS and the molecular structure is shown below. It has a broad spectrum of antifungal activity including pathogenic yeasts, dermatophytes, and Aspergillus spp., as well as Trichomonas vaginalis. Tioconazole is marketed as a topical formulation for the treatment of superficial dermatophyte infections and vaginal candidiasis. It is available without prescription in some countries.
Mechanism of action
In a similar manner to the other imidazole agents, tioconazole inhibits sterol biosynthesis by the inhibition of cytochrome P450-dependent C-14a-demethylation of lanosterol, preventing conversion to ergosterol, which results in depletion of normal fungal sterols (ergosterol) and accumulation of 14a-methyl sterols (lanosterol) in the fungal cell membrane. This inhibition is thought to produce fungistatic activity. At higher concentrations, tioconazole appears to produce direct cell membrane damage, resulting in leakage of essential intracellular components and cell death. Fungicidal activity has been demonstrated during stationary phases of growth in Candida spp. which supports the hypothesis of direct cell membrane damage.
Pharmacokinetics and Pharmacodynamics
Only minimal systemic absorption of tioconazole has been demonstrated in both animal and human studies. Following application of 0.1 g of 14C-labeled tioconazole 2% cream in the rat, percutaneous absorption was slow, with maximal plasma concentration achieved at 12 hours, and elimination of unchanged drug and metabolites was by urine (2.9%) and feces (16.8%) over 120 hours.
In one study, tioconazole was negligibly absorbed following topical application of the 2% cream or 28% nail solution to the skin twice daily for 14 days in a small number of patients. Plasma tioconazole levels were undetectable in half the patients and were 5–10 ng/ml in the remainder who applied the cream, and levels were undetectable in half the patients or detected at levels of 12–31 ng/ml in the remainder of those who applied the solution.
Absorption of tioconazole was also extremely low following intravaginal application of 2% cream daily for 30 days (peak plasma tioconazole concentration of 4–14 ng/ml), or a single 300-mg pessary (10–36 ng/ml). There was no detectable plasma tioconazole 24 hours after administration of the last dose. Mean concentrations of tioconazole in the vaginal fluid after a single application of 5ml 6% vaginal ointment (300 mg) were 104 mg/ml at 24 hours, 27 mg/ml at 48 hours, and 15 mg/ml at 72 hours. After intravaginal administration of a 300-mg pessary, the mean vaginal fluid tioconazole concentrations were 21.2 mg/ml at 24 hours and remained detectable in the majority of women at 48 hours, but not at 72 hours.
Toxicity
No systemic or laboratory adverse events have been noted in the clinical trials reported in the literature. Local skin reactions occur in 4–7% of patients. These are generally mild, transient, and include erythema, itching, and burning. Contact dermatitis, confirmed with patch testing, has been reported. Intravaginal application of tioconazole, whether in pessary, cream, or ointment formulation, results in leakage from the vagina.
In one study, a minority of women did not tolerate the leakage (2.5%) and no staining of clothing was reported. Reports of side-effects following intravaginal application are generally infrequent, and include mild burning, stinging, and pruritus. However, 30% of tioconazole-treated patients experienced local irritation or itching in one comparative study . In a few women with vaginal epithelial atrophy, there were reports of burning on urination following the use of intravaginal tioconazole. Tioconazole vaginal formulations have been used in the second and third trimester of pregnancy without evidence of drug-related complications.
- Related articles
- Related Qustion
Terconazole is a novel triazole ketal that was synthesized and developed by Janssen Pharmaceutica in 1983. It was the first triazole antifungal agent marketed. It has the chemical name cis-1-{p- [[2-(2,4-dichlorophenyl)-2-(1H-1,2,4-triazol-....
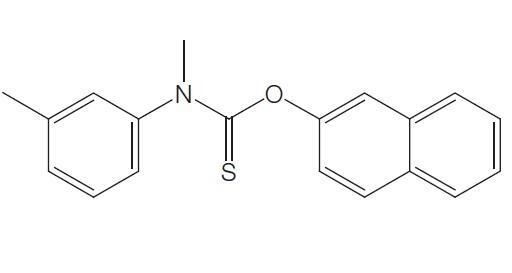
Mar 30,2022APITolnaftate is an old synthetic topical thiocarbamate antifungal agent with the chemical name 2-naphthyl N-methyl-N-(3-tolyl)thlonocarbamate; the chemical structure is shown below. Clinical use of tolnaftate has declined with the introductio....
Mar 30,2022APIVagistat
65899-73-2You may like
- Vagistat USP/EP/BP
-
- $1.10 / 1g
- 2025-11-18
- CAS:65899-73-2
- Min. Order: 1g
- Purity: 99.9%
- Supply Ability: 100 Tons Min
- Vagistat
-

- $0.00 / 25Kg/Drum
- 2025-11-18
- CAS:65899-73-2
- Min. Order: 1KG
- Purity: 99%-101%; EP
- Supply Ability: 500kg
- Vagistat
-

- $10.00 / 1KG
- 2025-11-14
- CAS:65899-73-2
- Min. Order: 1KG
- Purity: 99%
- Supply Ability: 10 mt