Properties, preparation and application of 1-cbz-4-piperidone
Jul 29,2022
General description
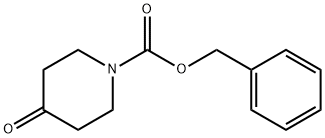
1-cbz-4-piperidone shows white to pale yellow. The CAS number of 1-cbz-4-piperidone is 19099-93-5, the molecular formula is C13H15NO3, the molecular Weight is 233.26, the melting point is 38-41°C, and the boiling point is 114-140 °C/0.25 mmHg (lit.). The density is 1.172 g/mL at 25 °C (lit.), the refractive index is n20/D 1.542(lit.), and the flash point is over 110 °C[1]. 1-cbz-4-piperidone is soluble in Chloroform, dichloromethane, ethyl Acetate, methanol. The pka is -1.63±0.20(Predicted).

Fig.1 The structure of 1-cbz-4-piperidone.
Systhesis
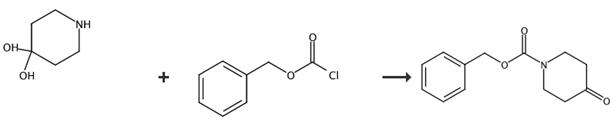
Fig. 2 The synthetic route of 1-cbz-4-piperidone[2].
To a solution of 4,4-piperidinediol hydrochloride (10 g, 65 mmol) in THF-H2O (160 mL, 1:1, 0.4 M) were added Na2CO3 (9.6 g, 91 mmol) and CbzCl (11 mL, 78 mmol). After stirring the mixture at room temperature for 9 h, the mixture was diluted with AcOEt and aqueous Na2CO3 (5% w/v). The aqueous layer was separated and extracted with AcOEt. The organic layers were combined, dried over MgSO4 and concentrated under reduced pressure. The residue was purified by flash column chromatography (AcOEt/hexane = 1/3 to 1/2). N-Cbz piperidone S29 (15 g, 65 mmol, 100%) as a colorless oil. 1H-NMR (400 MHz, CDCl3): δ 7.45-7.29 (m, 5H), 5.18 (s, 2H), 3.80 (t, J = 5.9 Hz, 4H), 2.46 (br s, 4H); 13C-NMR (100 MHz, CDCl3): δ 207.1, 155.1, 136.3, 128.6, 128.2, 128.0, 67.6, 43.1, 41.0; MS m/z: 233 (M+), 91 (100%); IR (neat, cm-1): 2964, 1698, 1429, 1229; HRMS: calcd. for C13H15NO3: 233.1052, found: 233.1055.
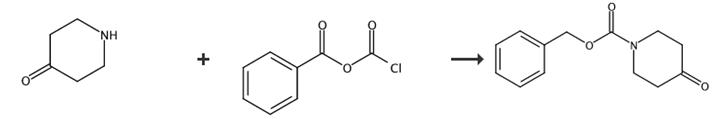
Fig. 3 The synthetic route of 1-cbz-4-piperidone[3].
4-Piperidone 10 (4 g, 26 mmol) was added to a solution of NaOH, 10%, and toluene cooled down at 0 °C. After a few minutes, a solution of benzyl chloroformiate (4.43 g, 26 mmol) in toluene was slowly added. The solution was allowed to warm to room temperature and was stirred for 2 h. The solvent was removed under reduced pressure, and the product was diluted in chloroform and washed with water (2×), with brine (2×) and dried over MgSO4. The solvent was removed under reduced pressure and the product was purified on silica gel column chromatography with EtOAc/heptane to give 11. 1-cbz-4-piperidone (5.88 g, 97%) 1H NMR (300 MHz, CDCl3): δ 7.36 (s, 5 H), 5.20 (s, 2H), 3.80 (t, J = 6.8 Hz, 4H), 2.46 (t, J = 6.8 Hz, 4H).
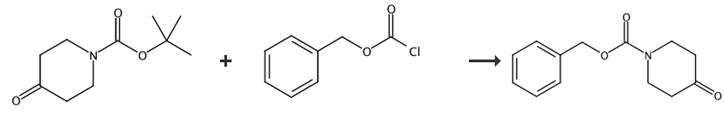
Fig. 4 The synthetic route of 1-cbz-4-piperidone[4].
To a solution of tert-butyl 4-oxopiperidine-1-carboxylate (2300 g, 11.6 mol) in 1,4-dioxane was added a solution of HCl (g)/1,4-dioxane (4 L, 10 mol/L) slowly at 0°C. After the addition, the reaction mixture was stirred for 4 h and TLC (EtOAc/petroleum ether = 1:5) showed the reaction was complete. The solvent was removed in vacuo to afford piperidin-4-one hydrochloride as a brown solid. Benzyl 4-oxopiperidine-1-carboxylate: To a stirred solution of piperidin-4-one hydrochloride (1567 g, 11.6 mol) and triethylamine (1400 g, 13.87 mol) in dichloromethane (12 L) was added benzyl chloroformate (1965 g, 11.55 mol) dropwise at 0°C. After the addition, the reaction mixture was allowed to warm to room temperature and stirred overnight. TLC (EtOAc/petroleum ether = 1:5) showed the reaction was complete. The mixture was washed with water (3 L) and brine (1 L), dried over sodium sulfate and concentrated in vacuo to give 1-cbz-4-piperidone as a colorless oil.
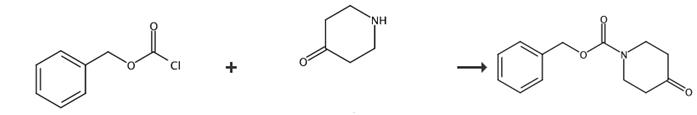
Fig. 5 The synthetic route of 1-cbz-4-piperidone[5].
To a stirred solution of piperidin-4-one hydrochloride (1567 g, 11.6 mol) and triethylamine (1400 g, 13.87 mol) in dichloromethane (12 L) was added benzyl chloroformate (1965 g, 11.55 mol) dropwise at 0°C. After the addition, the reaction mixture was allowed to warm to room temperature and stirred overnight. TLC (EtOAc/petroleum ether = 1:5) showed the reaction was complete. The mixture was washed with water (3 L) and brine (1 L), dried over sodium sulfate and concentrated in vacuo to give 1-cbz-4-piperidone as a colorless oil.
Application
Piperidine derivatives are a class of compounds with biological activities. Since the 20th century, many research groups have taken great interest in piperidine compounds and carried out some research[6-7]. In recent decades, many important achievements have been made in the development of piperidine derivatives. Many piperidine derivatives have been applied to the field of medicine, and a variety of piperidine drugs have been successfully developed, which has made outstanding contributions to the development of human health. Among many piperidone derivatives, 1-cbz-4-piperidone plays an important role in many fields. For example, in the field of medicine, it is often used to reduce patients' pain during and after surgery. 1-cbz-4-piperidone plays an important role in the treatment of analgesics[8-10].
In addition, the piperidone complex has an unusual use in the development of central nervous synthetics, This kind of medicine is developed on the basis of analgesic drugs, mostly used in the treatment of old sexual stupor, schizosis, depression and other diseases in the shu Shen Meridian [11]. This is because the piperidine ring introduced in the piperidine complex is directly connected to the dopamine receptor, which can promote the transformation of dopamine in the brain by blocking the dopamine receptor. It has good anti-excitability and anti-hallucination function. Some researchers have used pharmacological experiments to prove that 1-CBZ-4-Piperidone has the potential to treat alzheimer's disease and depression. The specific mechanism needs further stud[12].
Storage method
1-cbz-4-piperidone should be stored in a cool, dry place in a small, wellfilled, well-closed container, protected from light. When a partially filled container is used, the air should be replaced by nitrogen or another inert gas[13].
Hazard statement
Swallowing 1-cbz-4-piperidone is harmful to the body. It will also cause strong irritation to the skin, and even cause serious eye irritation. In addition, it may cause respiratory tract irritation.
References
[1] J. Henry Blackwell, G.R. Harris, M.A. Smith, M.J. Gaunt, Modular Photocatalytic Synthesis of α-Trialkyl-α-Tertiary Amines, J. Am. Chem. Soc. (2021) Ahead of Print.
[2] Y. Kuboki, M. Shiraki, Crystals of a cyclic amine derivative and pharmaceutical use thereof, Toray Industries, Inc., Japan . 2021, p. 37pp.
[3] Q. Lv, Y. Qi, B. Wang, Q. Chang, Preparation method of 2-(4-phenoxyphenyl)-6-(N-substituted oxycarbonylpiperidin-4-yl)nicotinamide, Xin Fa Pharmaceutical Co., Ltd., Peop. Rep. China . 2021, p. 12pp.
[4] C.G. Nasveschuk, R. Zeid, N. Yin, K.L. Jackson, G.K. Veits, M. Moustakim, J.L. Yap, Preparation of 3-substituted piperidine-2,6-dione compounds as targeted BRD9 degraders, C4 Therapeutics, Inc., USA . 2021, p. 779pp.
[5] J. Nugent, A.J. Sterling, N. Frank, J.J. Mousseau, E.A. Anderson, Synthesis of α-quaternary bicyclo[1.1.1]pentanes through synergistic organophotoredox and hydrogen atom transfer catalysis, Org. Lett. 23(21) (2021) 8628-8633.
[6] W.S. Palmer, J. Wu, S. Zipfel, K. Ozboya, D. Weiss, Preparation of bifunctional degraders of interleukin-1 receptor-associated kinases and therapeutic use thereof, Nurix Therapeutics, Inc., USA; Gilead Sciences, Inc. . 2021, p. 326pp.
[7] D. Reynolds, M.W. Seiler, A.A. Agrawal, F. Vaillancourt, P. Smith, Preparation of fused bicyclic compounds for modulating nucleic acid splicing, Remix Therapeutics Inc., USA . 2021, p. 218pp.
[8] H.P. Shunatona, G.P. Shearn-Nance, S.A. Mitchell, J. Buell, Bifunctional degraders of hematopoietic progenitor kinase and therapeutic uses thereof, Nurix Therapeutics, Inc., USA; Gilead Sciences, Inc. . 2021, p. 266pp.
[9] W. Su, T.-T. Wang, X. Tian, J.-R. Han, X.-L. Zhen, S.-M. Fan, Y.-X. You, Y.-K. Zhang, R.-X. Qiao, Q. Cheng, S. Liu, Stereoselective Dehydroxyboration of Allylic Alcohols to Access (E)-Allylboronates by a Combination of C-OH Cleavage and Boron Transfer under Iron Catalysis, Org. Lett. 23(23) (2021) 9094-9099.
[10] H. Takahashi, M. Shiraki, Crystals of cyclic amine derivative and pharmaceutical use thereof, Toray Industries, Inc., Japan . 2021, p. 31pp.
[11] L.-L. Wang, Y. Du, S.-M. Li, F. Cheng, N.-N. Zhang, R. Chen, X. Cui, S.-G. Yang, L.-L. Fan, J.-T. Wang, B. Guo, H.-S. Wu, J.-Q. Zhang, L. Tang, Design, synthesis and evaluation of tetrahydrocarbazole derivatives as potential hypoglycemic agents, Bioorg. Chem. 115 (2021) 105172.
[12] K.J. Wilson, S.E.R. Schiller, S. Negretti, Carboxamide compounds for the treatment of BAF complex-related diseases and their preparation, Foghorn Therapeutics Inc., USA . 2021, p. 354pp.
[13] X. Xu, J. Chen, G. Wang, X. Yang, Q. Li, Y. Zhang, C. Chen, X. Zhang, M. Qu, X. Zhou, Y. Wang, Y. Wang, X. Xia, Indole derivatives as TrkA receptor inhibitors and their preparation, pharmaceutical compositions and use in the treatment of cancer, Shanghai Synergy Pharmaceutical Sciences Co., Ltd., Peop. Rep. China; Zhejiang Huahai Pharmaceutical Co., Ltd. . 2021, p. 188pp.
- Related articles
- Related Qustion
Trometamol, also known as Tromethamine; 2-amino-2-(hydroxymethyl)-1,3-propanediol, is a white Chemicalbook-colored crystal or powder.....
Jul 29,2022Biochemical EngineeringTamoxifen citrate is an orally administered, nonsteroidal antiestrogen agent that is widely used for the treatment of breast cancer in recent years.....
Jul 29,2022API1-Cbz-4-Piperidone
19099-93-5You may like
1-Cbz-4-Piperidone manufacturers
- 1-Cbz-4-piperidone
-

- $10.00 / 1kg
- 2024-05-30
- CAS:19099-93-5
- Min. Order: 0.1kg
- Purity: 99%
- Supply Ability: 20ton