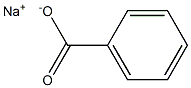
Sodium benzoate: Polarity; Functions and Applications
Jan 4,2024
Polarity of Sodium benzoate
Sodium benzoate is a polar chemical. This is due to the polar covalent bond between the carbon and oxygen atoms in the carbonyl group (-CO) and the carbon and oxygen atoms in the carboxyl group (-COO-).

Sodium benzoate is soluble in water because it is a salt of benzoic acid, which is a carboxylic acid. Carboxylic acids are usually soluble in water because they are able to form hydrogen bonds with water molecules. Water is a very polar molecule, so the negative end of the water molecule (the oxygen atom) is attracted to the positive sodium ion, while the positive end (the hydrogen atom) is attracted to the negatively charged COO- group. This weakens and dissociates the ionic bonds in sodium benzoate. This allows the sodium benzoate to dissolve and form a homogeneous solution with water. Sodium benzoate is soluble in water because it is very soluble in water, about 60 grams per 100 grams of water, depending on the temperature.
Functions and Applications
Sodium benzoate is a preservative. It is bacteriostatic and fungistatic under acidic conditions. It is used most prevalently in acidic foods such as salad dressings (vinegar), carbonated drinks (carbonic acid), jams and fruit juices (citric acid), pickles (vinegar), and condiments. It is also found in alcohol-based mouthwash and silver polish. It can also be found in cough syrups like Robitussin. sodium benzoate is also used for anticorrosive paper, latex paint, shoeshine, glue and fabric. sodium benzoate can also be used to make mordant in dyestuff industry, plasticizer in plastic industry and raw material for perfume industry. As a chemical reagent, it can be used as a cosolvent for serum bilirubin test.
- Related articles
- Related Qustion
- Sodium benzoate: Application, Pharmacokinetics, Toxicity Apr 17, 2023
Sodium benzoate is the sodium salt of benzoic acid, and is the first preservative allowed by the FDA for use in food products.
- What Is Sodium Benzoate? Oct 29, 2019
It’s an odorless, crystalline powder made by combining benzoic acid and sodium hydroxide. Benzoic acid is a good preservative on its own, and combining it with sodium hydroxide helps it dissolve in products (1).
4,4'-Methylenedianiline(MDA) is primarily used to produce 4,4-'methylenedianiline diisocyanate and other polymeric isocyanates which are used to manufacture polyurethane foams.....
Dec 10,2024Organic ChemistryTetrahydrofuran is a moderate polar aprotic solvent. THF has a moderate polarity thanks to the electronegativity difference between the oxygen and the carbon atoms.....
Jan 4,2024Organic reagentsSodium benzoate
532-32-1You may like
- Sodium benzoate
-

- $100.00/ kg
- 2025-02-08
- CAS:532-32-1
- Min. Order: 1kg
- Purity: 99%
- Supply Ability: 5000
- Sodium benzoate
-

- $6.00 / 1KG
- 2025-02-08
- CAS:532-32-1
- Min. Order: 1KG
- Purity: More than 99%
- Supply Ability: 2000KG/Month
- Sodium benzoate
-

- $6.00 / 1kg
- 2025-02-08
- CAS:532-32-1
- Min. Order: 1kg
- Purity: 99%
- Supply Ability: 2000KG/Month