Synthesis and Application of 6,6-Dimethyl-3-azabicyclo[3.1.0]hexane
Sep 8,2022
Physicochemical property
6,6-DiMethyl-3-azabicyclo[3.1.0]hexane has a boiling point of 135°C and it needs to be stored at 2-8°C and protected from light.

Fig. 1 The structure of 6,6-DiMethyl-3-azabicyclo[3.1.0]hexane.
Synthetic routes
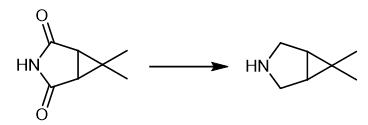
Fig. 2 The synthetic method 1 of 6,6-DiMethyl-3-azabicyclo[3.1.0]hexane.
A THF solution of LiAlH4 (500 mL, 2.4 M, 1.2 mol, 1.67 eq.) was charged into a 3-neck flask fitted with an N2 inlet. The contents of the flask were warmed to 40 °C while being purged with nitrogen. 100 g of III (0.72 mol, 1 eq.) and 400 mL of THF were added to a second flask and stirred until a clear solution was formed. The solution containing III in the second 3-necked flask containing was then added over an approximately 0.5 to 1 hour period to the reaction mixture containing LiAlH4 in the first 3-neck flask while allowing the temperature to rise to approximately 70°C (reflux). The second flask was rinsed with 100 mL of THF, which was added to the reaction mixture to ensure complete transfer of III. Upon completion of the addition of the solution, the reaction mixture was maintained at reflux temperature and stirred until the reaction was complete (approximately 3 hours). To a 3-necked flask fitted with a nitrogen inlet were charged 674 g of potassium sodium tartrate tetrahydrate (2.39 mol, 3.32 eq.) and 191 g of sodium hydroxide (4.78 mol, 6.64 eq.), 800 mL of H2O and 300 mL TBME. The mixture was agitated between 15 and 25°C for approximately 1 hour, or until all of the solids had dissolved. The reaction mixture was transferred via cannula to the biphasic quench mixture over approximately 10 to 20 minutes. The reaction flask was rinsed with 30 mL TBME which was also transferred via cannula to the quench flask. The biphasic mixture was agitated for an additional 15 to 30 minutes, and the layers were split at 40 °C. The aqueous layer was extracted twice with 100 mL TBME. The combined organic layers were fractionally distilled to yield IV as a colorless liquid (64.5 g, 88%). 1H NMR (CDCl3, 400 MHz): δ 3.07 (m, 2H), 2.89 (d, 2H, J= 11.6 Hz), 1.56 (br s. 1H), 1.25 (m, 2H), 1.00 (s, 3H), 0.98 (s, 3H) [1].
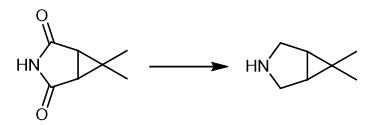
Fig. 3 The synthetic method 2 of 6,6-DiMethyl-3-azabicyclo[3.1.0]hexane.
To a well well-stirred suspension of 6,6-dimethyl-3-azabicyclo[3.1.0]hexane-2,4- dione of formula II, R1 is Hydrogen, (2.0 g, 14.38 mmol) in dry TI-IF (20.0 mL) sodium borohydride (1.64 g, 43.16 mmol) was added at - 30 to -20 °C. BF3 .bul. Et2O (43.16 mmol) was then added slowly at the same temperature. Reaction mixture was then stirred for 7-8 hrs at 40 to 50 °C temperature. THF was then distilled off followed by stripping of toluene to remove the THF. Reaction mass was cooled and unreacted NaBH4 was quenched with methanol (5.0 mL), diluted with water (100 mL), and extracted with toluene (3 x 25 mL). The combined toluene layers were washed with water and dried over anhydrous sodium sulfate. The solvent was removed under the reduced pressure keeping the temperature below 55°C to get 6, 6-dimethyl-3-azabicyclo [3.1.0] hexane as a with GC purity of 96%. colorless liquid, yield 1.3g [2].
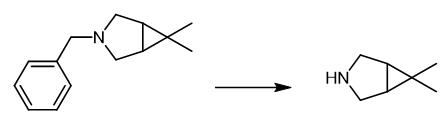
Fig. 3 The synthetic method 3 of 6,6-DiMethyl-3-azabicyclo[3.1.0]hexane.
To a round bottom flask was charged benzylated amine (179.5 g, 0.89 mol), MeOH (360 mL) and charcoal (5 g). The suspension was filtered and the cake washed with additional MeOH (50 ml). To the fiftrate was added acetic acid (90 mL) and the solution charged to a Buchi hydrogenator. To an additional flask was added 5% Pd/C (17.9 g, 10% w/w) and MeOH (30 mL). The mixture was added to the hydrogenator. The hydrogenator was charged with H2 (3 x 3 bar) and the resultant suspension agitated at 20-25 °C for 6 h. The catalyst was filtered and the hydrogenator washed with MeOH (2 x 100 mL). The combined solvents were concentrated in vacuo at 40 °C until the solution became viscous. To the solution was added water (120 mL) and the resultant mixture cooled to 20 °C. To this mixture was added 10 N NaOH (180 mL) and MTBE (450 mL). The biphasic mixture was agitated 10 min. and allowed to settle an additional 10 min. The organic phase was heated to 90 °C and concentrated at atmosphere. The resultant crude residue was distilled at 130 mm Hg to give the compound of Formula III (72.8 g, 97.8%) as a colorless liquid [3].
Application
Intermediates of Nirmatrelvir
The severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) 3C-like protease inhibitor PF-07321332 (nirmatrelvir), in combination with ritonavir (Paxlovid), was recently granted emergency use authorization by multiple regulatory agencies for the treatment of coronavirus disease 2019 (COVID-19) in adults and pediatric patients. Disposition studies on nirmatrelvir in animals and in human reagents, which were used to support clinical studies, are described herein. Plasma clearance was moderate in rats (27.2 ml/min per kg) and monkeys (17.1 ml/min per kg), resulting in half-lives of 5.1 and 0.8 hours, respectively. The corresponding oral bioavailability was moderate in rats (34%-50%) and low in monkeys (8.5%), primarily due to oxidative metabolism along the gastrointestinal tract in this species. Nirmatrelvir demonstrated moderate plasma protein binding in rats, monkeys, and humans with mean unbound fractions ranging from 0.310 to 0.478. The metabolism of nirmatrelvir was qualitatively similar in liver microsomes and hepatocytes from rats, monkeys, and humans; prominent metabolites arose via cytochrome P450 (CYP450)-mediated oxidations on the P1 pyrrolidinone ring, P2 6,6-dimethyl-3-azabicyclo[3.1.0]hexane, and the tertiary-butyl group at the P3 position. Reaction phenotyping studies in human liver microsomes revealed that CYP3A4 was primarily responsible (fraction metabolized 5 0.99) for the oxidative metabolism of nirmatrelvir. Minor clearance mechanisms involving renal and biliary excretion of unchanged nirmatrelvir were also noted in animals and in sandwich-cultured human hepatocytes. Nirmatrelvir was a reversible and time-dependent inhibitor as well as inducer of CYP3A activity in vitro. First-in-human pharmacokinetic studies have demonstrated a considerable boost in the oral systemic exposure of nirmatrelvir upon coadministration with the CYP3A4 inhibitor ritonavir, consistent with the predominant role of CYP3A4 in nirmatrelvir metabolism. SIGNIFICANCE STATEMENT The manuscript describes the preclinical disposition, metabolism, and drug-drug interaction potential of PF-07321332 (nirmatrelvir), an orally active peptidomimetic-based inhibitor of the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) 3CL protease, which has been granted emergency use authorization by multiple regulatory agencies around the globe for the treatment of coronavirus disease 2019 (COVID-19) in COVID-19-positive adults and pediatric patients who are at high risk for progression to severe COVID19, including hospitalization or death [4].
References
[1] Wu G, Chen F X, Rashatasakhon P, et al. Process for the preparation of 6,6-dimethyl-
- Related articles
- Related Qustion
You may like
6,6-DiMethyl-3-azabicyclo[3.1.0]hexane Boceprevir Key interMediate manufacturers
- 6,6-Dimethyl-3-azabicyclo[3.1.0]hexane
-
![943516-54-9 6,6-Dimethyl-3-azabicyclo[3.1.0]hexane](/ProductImageEN/2022-03/Small/92b2b4fc-940e-49a0-871d-92c5c9a2b00b.jpg)
- $0.00 / 1kg
- 2025-12-19
- CAS:943516-54-9
- Min. Order: 1kg
- Purity: 99%
- Supply Ability: 20t
- 6,6-Dimethyl-3-azabicyclo[3.1.0]hexane
-
![943516-54-9 6,6-Dimethyl-3-azabicyclo[3.1.0]hexane](/ProductImageEN1/2024-11/Small/f453bd5a-1619-407b-a269-bc92abdd482c.png)
- $0.00 / 25KG
- 2025-12-01
- CAS:943516-54-9
- Min. Order: 1KG
- Purity: 98%
- Supply Ability: 1000KGS
- 6,6-DiMethyl-3-azabicyclo[3.1.0]hexane Boceprevir Key interMediate
-
![943516-54-9 6,6-DiMethyl-3-azabicyclo[3.1.0]hexane Boceprevir Key interMediate](/ProductImageEN/2022-02/Small/6bc32713-e9ed-47e4-9407-2a48c67f77c7.jpg)
- $1.10 / 1g
- 2025-11-18
- CAS:943516-54-9
- Min. Order: 1g
- Purity: 99.0% Min
- Supply Ability: 100 Tons






