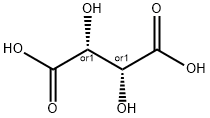
Tartaric Acid, DL-Tartaric Acid, d-tartaric Acid, and l-tartaric Acid
Sep 12,2024
Tartaric Acid
Tartaric Acid (2,3-dihydroxybutanedioic Acid) is a naturally occurring dicarboxylic acid containing two stereocenters. It is a molecule capable of existing in at least three different forms: the neutral bi-acid form; the monotartrate form, where one of the carboxylic acid groups has deprotonated to give a carboxylate moiety; and the bitartrate form, in which both acid groups have deprotonated. Furthermore, the molecule chemistry is complicated by the fact that protonated carboxylic acid groups are possibly involved in hydrogen bonds with other acid and alcohol groups, which potentially lead to several intermolecular interactions.

Tartaric Acid is a historical compound, dating back more than 160 years to when Louis Pasteur separated it into two enantiomers with a magnifying lens and a pair of tweezers.
Three stereoisomeric forms
Three stereoisomeric forms of tartaric Acid exist (1) dextrorotatory tartaric Acid (d-tartaric Acid) found in grapes and several other fruits, (2) levorotatory tartaric Acid (l-tartaric Acid) obtained chiefly by resolution of racemic tartaric Acid, and (3) a meso or achiral form. Racemic tartaric Acid (an equal mixture of d- and l-tartaric Acid) is prepared commercially by the molybdenum- or tungsten-catalyzed oxidation of maleic anhydride with hydrogen peroxide. DL-Tartaric Acid is a food additive. It is a white, crystalline powder that is stable in the air and soluble in water, ethanol, and ethyl ether. It is used in the manufacturing of tartrates and in the production of electroplates, chemical fertilizers, glass, foods, pharmaceuticals, etc.
It is manufactured from potassium hydrogen tartrate (wine tartar, cream of tartar – a by-product of the wine-making industry) via calcium salt. (S, S)-Tartaric Acid is also available commercially; it can be obtained from the racemic Acid by several resolution procedures or d-xylose. The highly functionalized and C2-symmetric tartaric acid molecule is perfectly tailored for applications as a resolving agent and chiral ligand. In fact, tartaric Acid is the most frequently used resolving agent for racemic amines.
- Related articles
- Related Qustion
- DL-Tartaric acid: Synthesis and Stereochemistry Mar 12, 2025
DL-Tartaric acid (racemic mixture) is a synthetic equimolar combination of L(+) and D(-) forms and is optically inactive due to internal compensation.
- Application and Preparation of DL-Tartaric Acid Aug 8, 2022
DL-tartaric acid is a mixture of equal amounts of L-TA and D-TA, which is widely used in food, medicine, chemical industry, light industry and other industries.
1,7-Dimethylxanthine is a naturally occurring alkaloid compound that can enhance alertness and reduce drowsiness.....
Feb 27,2025API1-BOC-Piperazine is essential for synthesizing bioactive compounds, with recent methods using diethylamine enhancing efficiency, yield, purity, and environmental sustainability.....
Sep 12,2024APIDL-Tartaric acid
133-37-9You may like
DL-Tartaric acid manufacturers
- DL-Tartaric acid
-

- $1.00 / 1g
- 2025-10-31
- CAS:133-37-9
- Min. Order: 1g
- Purity: 99%
- Supply Ability: 1000kg
- DL-Tartaric acid
-

- $0.00 / 1kg
- 2025-10-31
- CAS:133-37-9
- Min. Order: 1kg
- Purity: 99%
- Supply Ability: 1000
- DL-Tartaric acid
-

- $99.00/ kg
- 2025-10-29
- CAS:133-37-9
- Min. Order: 0.001kg
- Purity: 99%
- Supply Ability: 5000