The crystal structure of uranium dicarbide
Mar 22,2024
Uranium carbide (UC) and uranium dicarbide (UC2) are two carbides of uranium which can be used as nuclear fuels. Uranium sesquicarbide (U2C3) is another carbide of uranium. U2C3 cannot be manufactured through casting or compaction of a powder. However, UC2 may transform into U2C3 at high temperatures and under stress.
UC2 may be considered an intermetallic compound of U and C2, where the C-C bond in the neutral C2 molecule is a double bond, while the observed C-C distance in UC2 equals the C-C double bond distance[1].
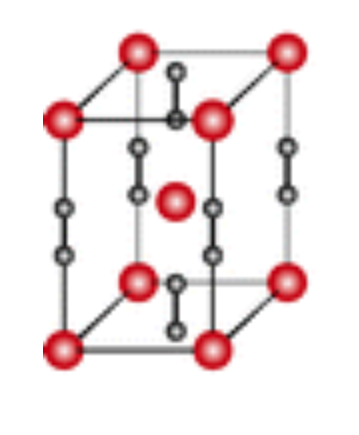
The structure of a-UC2 has been determined by X-ray and neutron diffraction to be the CaC2-type body-centered tetragonal. Above 1,765℃ the tetragonal structure transforms to a cubic structure, which is considered to be of the NaC1-type with C2 group as in the case of KCN. However, cubic UC2 has never been obtained at room temperature, even if quenched, because the polymorphic transition between a- and p-phase is too rapid to be frozen. Various lattice parameters for a-UC2 have been given in the literature. The reported data range between ao=3.515Å, co= 5.98Å, and a0=3.526Å, C0=6.00Å, and the composition from UC1-86 to UCl-96[2].
The structure of the binary dicarbide UC2 has been determined several times at various temperatures using powder neutron diffraction methods, revealing a tetragonally distorted NaCl-type structure from 5–2038 K that is analogous to the structure of CaC2. At 5 K, UC2 displays a C–C distance of 1.346(1)Å, a U–C distance of 2.321(1)Å along the tetragonally distorted c-axis containing the linear (–U–C2–U–C2–)• array, and a U–C distance of 2.576(1)Å connecting the C2 unit to the uranium centers occupying the adjacent interstices. The notably long C–C distance in UC2 has been explained as a consequence of the C–C p* -orbital being electronically populated as a participant in an occupied conduction band or by viewing UC2 as a structurally ordered alloy of U0 and neutral doubly-bonded C2 units[3].
References
[1] Fox, Alexander R. Sidney E. Creutz and Christopher C. Cummins. “A bimetallic uranium μ-dicarbide complex: synthesis, X-ray crystal structure, and bonding†.” Dalton Transactions 29 (2010): 6632–6634.
[2] M. Atoji, R. C. Medrud. “Structures of Calcium Dicarbide and Uranium Dicarbide by Neutron Diffraction.” Journal of Chemical Physics 77 1 (1959): 332–337.
[3] H. Tagawa, Y. Sasaki, K. Fujii. “Studies on the Uranium Dicarbide Phase.” Journal of Nuclear Science and Technology 8 1 (1971): 244–249.
- Related articles
- Related Qustion
Supplementation with pyridoxal 5'-phosphate monohydrate can synthesize neurotransmitters such as dopamine and serotonin, maintaining a healthy nervous system.....
Nov 4,2025Biochemical EngineeringNeoprene is widely used in the manufacture of industrial rubber products such as wire and cable sheaths, oil-resistant hoses and rubber plates, conveyor belts, rubberized fabrics and various sealing ring gaskets.....
Mar 22,2024APIuranium dicarbide
12071-33-9You may like






