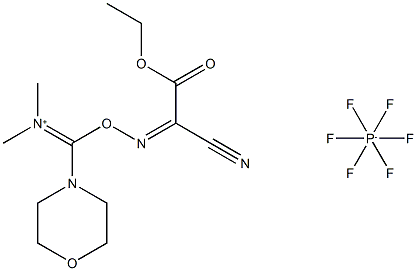
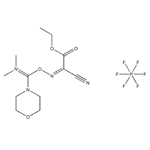
The process of COMU coupling reaction.
Dec 4,2023
Description
1-[(1-(Cyano-2-ethoxy-2-oxoethylideneaminooxy)-dimethylamino-morpholinomethylene)] methanaminium hexafluorophosphate (COMU), a uronium-type coupling reagent, is the most influential representative of the Oxyma-based family of coupling reagents and is now commercially available from numerous suppliers[1]. It can be rapidly accessed from dimethylcarbamoyl chloride and Oxyma in a few steps.
Using COMU without excess base is an attractive methodology to reduce the degree of stereomutation in peptide couplings, which is a dramatic side reaction with severe implications in peptide purity and activity. The characteristic of COMU is that the course of reaction can be followed due to a change of color, depending on the type of base used. Once the reaction is complete, the solution becomes colorless to yellow, again depending on the type of base used.
General procedure for coupling reaction using COMU in solution-phase
COMU (0.25 mmol) was added to a mixture of the N-protected amino acid (0.25 mmol), the amino component (0.25 mmol), and base (0.50 mmol or 0.75 mmol in case of ester hydrochloride) in DMF (2 ml) at 0 ℃, and the reaction mixture was stirred at 0 ℃ for 1 h and at r.t. for 2–3 h. The mixture was diluted with EtOAc (25 ml) and extracted with 1 N HC1 (2 × 5 ml), 1 N NaHCO3 (2 × 5 ml) and saturated NaCl (2 × 5 ml). The EtOAc was then dried with MgSO4, the solvent was removed, and HPLC and NMR directly analyzed the crude peptide[2].
General procedure for coupling reaction using COMU in solid-phase
N-Protected amino acid (3 equiv.), base (6 equiv.), and COMU (3 Equiv.) were pre-activated in DMF (0.3 M) for 1 min and then added to the amino resin with manual stirring for 2–5 min and allowed to stand at r.t. for 10–30 min (1 h for hindered residues or 1 h double coupling, also). The resin was filtered and washed with DMF.
References
[1] Ramon Subirós-Funosas. “COMU: scope and limitations of the latest innovation in peptide acyl transfer reagents.” Journal of Peptide Science 19 7 (2013): 408–414.
[2] Ayman El-Faham, Fernando Albericio. “COMU: A third generation of uronium-type coupling reagents.” Journal of Peptide Science 16 1 (2009): 6–9.
- Related articles
- Related Qustion
- An efficient Coupling Reagent: COMU Dec 4, 2023
COMU is a more efficient alternative to classical benzotriazole immonium salts regarding racemization suppression, coupling effectiveness, stability, and solubility.
Camphor remains a product with the potential for serious toxicity in the pediatric patient. One mouthful (9.3 mL) of several currently available products has the potential to cause serious morbidity and even death.....
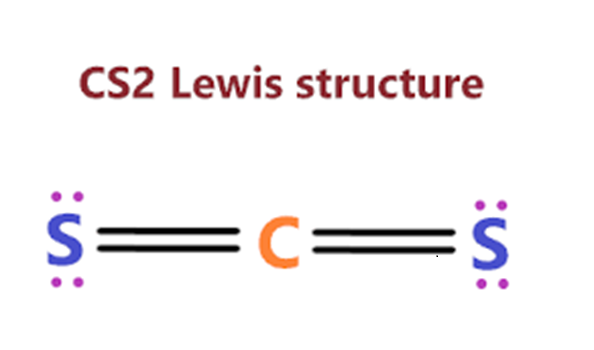
Dec 4,2023APIThe CS2 Lewis structure consists of a central carbon atom (C) and two external sulphur atoms (S) bonded at 180°.....
Dec 4,2023APICOMU
1075198-30-9You may like
- COMU
-
- $0.00 / 1KG
- 2024-11-14
- CAS:1075198-30-9
- Min. Order: 1KG
- Purity: 98%min
- Supply Ability: 30tons/month
- COMU
-
- $0.00 / 1KG
- 2022-09-24
- CAS:1075198-30-9
- Min. Order: 1KG
- Purity: 98%
- Supply Ability: 1ton
- COMU
-

- $15.00 / 1KG
- 2021-07-13
- CAS:1075198-30-9
- Min. Order: 1KG
- Purity: 99%+ HPLC
- Supply Ability: Monthly supply of 1 ton