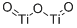The research on corundum-type Ti2O3
May 10,2024
Description
Generally, Titanium(III) oxide (Ti2O3) has a corundum structure in bulk with an ultra-narrow bandgap (Eg ≈ 0.1 eV), exhibiting excellent photothermal effect and mid-infrared photodetection[1].
Structure
Titanium(III) oxide is Corundum structured and crystallizes in the trigonal R̅3c space group. Ti³⁺ is bonded to six equivalent O²⁻ atoms to form a mixture of edge, corner, and face-sharing TiO₆ octahedra. The corner-sharing octahedral tilt angles range from 47-56°. Three shorter (2.01 Å) and three longer (2.07 Å) Ti-O bond lengths exist. O²⁻ is bonded to four equivalent Ti³⁺ atoms to form a mixture of distorted edge and corner-sharing OTi₄ trigonal pyramids.
Ti2O3 nanoparticle
the reaction of the rutile nanoparticles with CaH2 at 350 °C for several days yielded corundum-type Ti2O3, keeping the nanomorphology of the precursor. This is the first example of Ti2O3 nanoparticles, but its bulk or sintered sample has been extensively studied over the last half century because a sluggish I-M transition takes place in the temperature range from 420 to 550 K[2].
As for Ti2O3, each titanium ion is subject to a slight trigonal distortion with six oxygen anions, which splits 3-fold degenerate t2g orbitals into a low-lying a1g orbital directed along the c axis and two egπ orbitals directed to near-neighbor Ti ions in the basal plane. The strong hybridization between two a1g orbitals within the Ti(1)–Ti(2) bond yields the bonding (a1g) and antibonding (a*1g) bands with a large energy gap. In contrast, the egπ and egπ* bands are partially overlapped and located between the a1g and a*1g bands. In the insulating state, a small energy gap of 0.1∼0.2 meV opens between the fully occupied a1g and unoccupied egπ bands. The mechanism of the I-M transition of Ti2O3 is still controversial. However, a widely accepted view is that a gradual increase of the Ti(1)–Ti(2) length on heating suppresses the separation of the a1g and a*1g bands, finally leading to the breakdown of the semiconducting gap between a1g and egπ bands. The Ti(1)–Ti(3) lengths in the basal plane shrink slightly across the transition.
Ti2O3 nanoparticles revealed the short a and extended c lattice parameters (a = 5.0745 Å, c = 13.7516 Å) compared with those of bulk Ti2O3 (a = 5.1570 Å, c = 13.610 Å) [31]. The color was bluish-black for the nanoparticles but dark brown for the bulk.
References
[1] Yangyang Li. “Electronic-reconstruction-enhanced hydrogen evolution catalysis in oxide polymorphs.” Nature Communications 10 1 (2019): 3149.
[2] Yoshihiro Tsujimoto . “Size dependence of structural, magnetic, and electrical properties in corundum-type Ti2O3 nanoparticles showing insulator–metal transition.” Journal of Asian Ceramic Societies 3 3 (2015): Pages 325-333.
- Related articles
- Related Qustion
Mesityl Oxide, with its stable structure and low reactivity, can undergo substitution reactions and efficient absorption, allowing quantification via gas chromatography in pharmacokinetics.....
May 10,2024APIEverolimus is an immunosuppressant-targeted anticancer drug that can be used for a number of different types of cancers.....
May 10,2024DrugsTitanium(III) oxide
1344-54-3You may like
- What is nitrogen used for?
May 24, 2024
- The structure of Aluminum oxide
May 24, 2024
- The brief introduction of Lead selenide
May 23, 2024
Titanium(III) oxide manufacturers
- Dititanium trioxide
-

- $1.00 / 1KG
- 2019-07-10
- CAS:1344-54-3
- Min. Order: 1KG
- Purity: 99%
- Supply Ability: 100kg