The Synthesis method of Esaxerenone
Jan 5,2024
Description
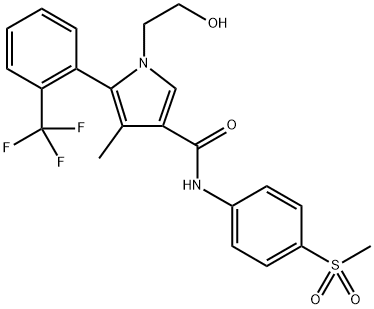
Esaxerenone, a novel, nonsteroidal, selective mineralocorticoid receptor antagonist (MRA) discovered by Exelixis and developed by Daiichi Sankyo, was approved in Japan to treat hypertension. Excessive mineralocorticoid receptor activation by endogenous ligands (e.g., aldosterone) is involved in hypertension[1]. Esaxerenone exerts an antihypertensive effect by blocking the activation of this receptor. The drug has at least a 1000-fold higher selectivity for the mineralocorticoid receptor than other steroid hormone receptors, a long half-life, high oral bioavailability, and a more pronounced antihypertensive effect than spironolactone or eplerenone.
Synthesis method
A method to generate the esaxerenone atropisomer without column chromatography is reported by researchers at Daiichi Sankyo, as depicted below. Ketone 60 underwent α-bromination followed by a reaction with the enolate of ethyl cyanoacetate to give cyanoketone 61, carried forward in toluene. Pyrrole formation occurred under modified Knorr conditions to give mixtures of 62 and its 2-chlorinated counterpart, which was dechlorinated under transfer hydrogenation conditions to give pyrrole 62 in 82% yield from ketone 60. N-Alkylation of the pyrrole with ethylene carbonate and subsequent ester hydrolysis generated acid 63 as a mixture of atropisomers. Ultimately, heating a solution of 63 and quinine in dimethylacetamide, ethyl acetate, and water followed by gradual cooling provided the desired S atropisomer quinine salt 64 in 43% yield with 98% de.
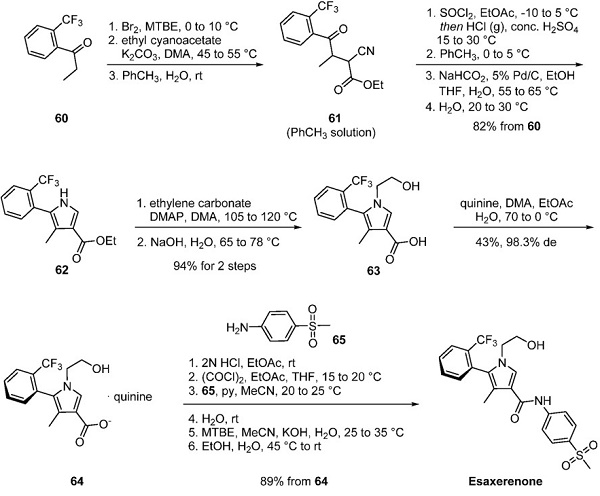
Interestingly, the identity of the desired atropisomers was initially confirmed by X-ray crystallography, and subsequently, an HPLC method was disclosed by Daichi Sankyo that described the differentiating characterization of the atropisomers within their 2016 patent. The corresponding free base carried forward as a solution in ethyl acetate, was converted to acid chloride using oxalyl chloride and reacted with 4-(methylsulfonyl)aniline (65). The free alcohol in intermediate 64 was concomitantly reacted with oxalyl chloride and aniline 65 to form the corresponding oxamate intermediate. Hydrolysis of the oxamate with potassium hydroxide and crystallization of the product from ethanol and water cleanly provided esaxerenone an 89% yield from 64.
References
[1] Duggan, Sean. “Esaxerenone: First Global Approval.” Drugs 79 4 (2019): 477–481.
[2] Andrew C. Flick. “Synthetic Approaches to the New Drugs Approved during 2019.” Journal of Medicinal Chemistry 64 7 (2021): 3604–3657.
- Related articles
- Related Qustion
Selumetinib Sulfate was prepared by a two-step chemical reaction by first synthesising Selumitinib Benzimidazole intermediates using 2,3,4-trifluorobenzoic acid as starting material.....
Jan 5,2024InhibitorsSurufatinib is synthesised from dichloropyrimidine and hydroxyindole by substitution reaction. This process requires the participation of Surufatinib Amine.....
Jan 5,2024Inhibitors