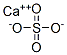What is the charge of calcium sulfate?
Feb 6,2024
Composition of calcium sulphate
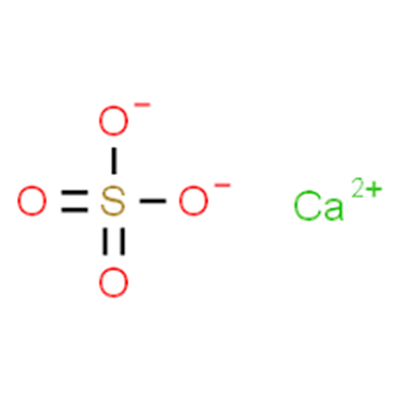
Calcium sulphate is a compound formed by the combination of calcium ion and sulphate ion. Calcium ion carry two positive charge (Ca2+) whereas sulphate ion carry two negative ion(SO42−), both combine to form a neutral compound CaSO4.
(I) Chemical formula represents number of atoms of elements present in a compound. Chemical formula is given by symbol of element and number of atoms of the element.
Ca2(SO4)3 is overall neutral.
⇒ 2 × ( 2 ) + 3 × ( − 2 ) = 0 [ Ca ⟶ + 2 valency, SO4⟶ − 2 valency]
⇒ 4 − 6 = 0
∴ − 2 charge would be more on Ca2(SO4)3 if this chemical formula of calcium sulphate is assumed.
Correct chemical formula is given as (Refer to Image):
(II) Insoluble anhydrite CaSO4 has been commercially manufactured by the calcination of gypsum rock at a relatively high temperature.

Hence, both statement are incorrect.

Related Research
Ion retention in variable-charge soils can be enhanced by the presence of certain ions with opposite charge, thereby influencing the movement of these ions through the soil profile. Studies examining these interactions are still incipient, however, especially regarding its modeling. We present results from batch and miscible displacement experiments describing Ca 2+ and SO4 2- movement in a variable-charge soil from New Zealand. Evidence was found for ion-pair adsorption (IPA) of both Ca 2+ and SO4 2- The results were modeled using the convection-dispersion equation (CDE), coupled with two different mathematical approaches proposed to account for IPA. The first approach related IPA to the single soil adsorption capacity, which is governed by particle-surface phenomena. For the second approach, IPA was related solely to the soil solution concentration. Both these approaches described the adsorption data from the batch experiment reasonably well, as well as the breakthrough curves from the miscible displacement experiments. The first approach showed better overall agreement. Significant differences were found, however, when the adsorption parameters were identified by fitting models to data from either batch or miscible displacement experiments. Although more studies are needed to better understand IPA, our results showed that the extent of IPA can be large and it should not be ignored when predicting SO4 2- and Ca 2+ movement in variable-charge soils.
References:
[1] R. CICHOTA. Simultaneous Adsorption of Calcium and Sulfate and Its Effect on Their Movement[J]. Soil Science Society of America Journal, 2007. DOI:10.2136/SSSAJ2006.0206.
- Related articles
- Related Qustion
- The CaSO4 – H2O System: Physical properties and Laboratory Synthesis Mar 16, 2024
The CaSO4– H2O system is characterized by five solid phases.
- Application of calcium sulfate Mar 8, 2022
Calcium sulfate (CaSO4), also known as alabaster, gypsum, and plaster of paris, can be obtained by mining natural reservoirs or by chemical synthesis. Naturally occurring calcium sulfate has GRAS status and is most commonly used as a dough
Supplementation with pyridoxal 5'-phosphate monohydrate can synthesize neurotransmitters such as dopamine and serotonin, maintaining a healthy nervous system.....
Nov 4,2025Biochemical EngineeringStanozolol aids in treating certain dermatological conditions but requires cautious use due to potential hepatic and androgenic side effects.....
Feb 7,2024APIcalcium sulfate
99400-01-8You may like
- CALCIUM SULFATE
-

- 2025-12-20
- CAS:99400-01-8
- Min. Order:
- Purity: 0.99
- Supply Ability:
- calcium sulfate
-

- $1.10 / 1g
- 2025-11-18
- CAS:99400-01-8
- Min. Order: 1g
- Purity: 99.9% Min
- Supply Ability: 100 Tons
- calcium sulfate
-

- $0.00 / 1KG
- 2025-06-27
- CAS:99400-01-8
- Min. Order: 1KG
- Purity: 99%
- Supply Ability: 500000kg