Бензилхлорид химические свойства, назначение, производство
Химические свойства
Benzyl chloride is a colorless to s lightly yellow liquid with a strong, unpleasant, irritating odor. The Odor Threshold is 0.05 ppm.The stabilized form of benzyl chloride contains a fixed amount of a sodium carbonate solution or propylene oxide.
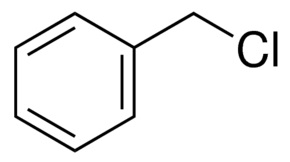
Физические свойства
Colorless to pale yellowish-brown liquid with a pungent, aromatic, irritating odor. Odor threshold concentration is 47 ppbv (Leonardos et al., 1969). Katz and Talbert (1930) reported an experimental detection odor threshold concentration of 210 μg/m3 (41 ppbv). The solubility of benzyl chloride in water is 0.33 g/L at 4°C, 0.49 g/L at 20°C, and 0.55 g/L at 30°C. It is freely soluble in chloroform, acetone, acetic acid esters, diethyl ether, and ethyl alcohol.
Использование
Benzyl chloride is used in the manufacture of benzyl Compounds, dyes, artificial resins, tanning agents, phar maceuticals, plasticizers, synthetic tannins, perfumes, lubricants, and quaternary ammonium compounds. It is also an intermediate in the preparation of phenylacetic acid (precursor to phamaceuticals).
Методы производства
Benzyl chloride can be synthesized by chloromethylation of benzene in the presence of a catalyst (ZnCl2) or by treatment of benzyl alcohol with SO2Cl2. Commercially it is produced by chlorination of boiling toluene in the presence of light.
прикладной
Benzyl chloride is used mainly to produce plasticizers (e.g., benzyl butyl phthalate), benzyl alcohol, and phenylacetic acid via benzyl cyanide (used in the production of synthetic penicillin). On a smaller scale, it is used to produce quaternary ammonium salts (for disinfectants and phase-transfer catalysts), benzyl esters (benzyl benzoate and benzyl acetate for the flavors and perfumes industry), dyes of the triphenylmethane series, dibenzyl disulfide (antioxidant for lubricants), benzylphenol, and benzylamines.
Определение
ChEBI: Benzyl chloride is a member of the class of benzyl chlorides that is toluene substituted on the alpha-carbon with chlorine.
Подготовка
Benzyl chloride is the starting material for a large number of industrial syntheses. The first preparation of it involved not the chlorination of toluene, however, but the reaction of benzyl alcohol with hydrochloric acid (S. Cannizzaro, 1853). Today, most side-chain chlorination of toluene to produce benzyl chloride. Dibenzyl ether is formed as a byproduct in the alkaline hydrolysis of benzyl chloride to benzyl alcohol. This ether can be re-converted to benzyl chloride by cleavage with hydrogen chloride at a temperature below 100°C.
Реакции
Benzyl chloride can be oxidized to benzoic acid or benzaldehyde, or substituted to give the halogenated, sulfonated or nitrated product.With NH3 it yields mono-, di- or tribenzyl amine. With alcohols in base the benzylalkyl ether is formed. With phenols either the phenolic or nuclear hydrogens can react to give benzylaryl ether or benzylated phenols. Reaction with NaCN gives benzyl cyanide (phenylacetonitrile); with aliphatic primary amines the product is the N-alkylbenzylamine, and with aromatic primary amines N-benzylaniline is formed. Benzyl chloride is converted to butyl benzyl phthalate plasticizer and other chemicals.
Общее описание
Benzyl chloride appears as a colorless liquid with an irritating odor. Toxic by inhalation and skin absorption. Flash point 153°F. Slightly soluble in water. Corrosive to metals and tissue. A lachrymator. Density 9.2 lb /gal.
Реакции воздуха и воды
A lachrymator. Slightly soluble in water.
Профиль реактивности
Halogenated aliphatic compounds, such as Benzyl chloride, are moderately or very reactive. Reactivity generally decreases with increased degree of substitution of halogen for hydrogen atoms. Materials in this group are incompatible with strong oxidizing and reducing agents. Also, they are incompatible with many amines, nitrides, azo/diazo compounds, alkali metals, and epoxides.
Опасность
Highly toxic, intense eye and skin irritant. A
lachrymator. Upper respiratory tract irritant. Prob-
able carcinogen.
Угроза здоровью
Benzyl chloride is a corrosive liquid. Con tact with the eyes can cause corneal injury.Exposure to its vapors can produce intenseirritation of the eyes, nose, and throat. Highconcentrations may cause lung edema anddepression of the central nervous system.Flury and Zernik (1931) stated that exposureto 16 ppm for 1 minute was intolerable tohumans. The LC50 values for a 2-hour expo sure in mice and rats are 80 and 150 ppm,respectively. The subcutaneous LD50 valuein rats is 1000 mg/kg (NIOSH 1986).
Benzyl chloride tested positive to thehistidine reversion–Ames test for mutagenic ity. Subcutaneous administration of this com pound in laboratory animals caused tumors atthe site of application.
Влияние на здоровье
A concentration of 16
ppm of benzyl chloride in air is reported to be
intolerable to humans within 1 min. The compound is a potent lachrymator, strongly irritating
to the eyes, nose, and throat and capable of
causing lung edema.
Пожароопасность
Benzyl chloride burns but does not ignite readily. Benzyl chloride may ignite combustibles. When heated to decomposition, Benzyl chloride emits toxic and corrosive fumes. Some organic chlorides decompose to yield phosgene. Incompatible with active metals such as copper, aluminum, magnesium, iron, zinc, and tin and keep from strong oxidizing agents. Avoid contact with acids or acid fumes. Keep separate from oxidizing materials. May become unstable at elevated temperatures and pressures; may react with water resulting in some nonviolent release of energy. Polymerizes with evolution of heat and hydrogen chloride when in contact with all common metals except nickel and lead.
Токсикология
The acute oral toxicity (LD50) of benzyl chloride
in rats is 1231 mg/kg and in mice 1624 mg/kg. The subcutaneous LD50 (in rats) of benzyl
chloride in oil solution is 1000 mg/kg.
Exposure of rats and mice to benzyl chloride
concentrations of 100 –1000 mg/m3 for 2 h
caused irritation of the mucous membranes and
conjunctivitis, vol. 11. Benzyl chloride is a
strong skin-sensitizing agent for guinea pigs . Benzyl chloride acts weakly mutagenic
in validated test systems.
Subcutaneous injection of weekly doses of 80
mg/kg for 1 year followed by a post-observation
period resulted in local sarcomas with lung
metastases in rats. The mean induction time was
500 d. After dermal application of benzyl
chloride, skin carcinomas were observed in mice.
Возможный контакт
Used as an intermediate and as an irritant gas in chemical warfare. In contrast to phenyl halides, benzyl halides are very reactive. Benzyl chloride is used in production of benzal chloride, benzyl alcohol, and benzaldehyde. Industrial usage includes the manufacture of benzyl compounds, cosmetics, dyes, plastics, synthetic tannins, perfumes and resins. It is used in the manufacture of many pharmaceuticals. Suggested uses of benzyl chloride include: the vulcanization of fluororubbers and the benzylation of phenol and its derivatives for the production of possible disinfectants.
Канцерогенность
Benzyl chloride caused genetic mutations
and chromosome-damaging effects in a wide
variety of in vitro assays; it was not mutagenic
in vivo in the mouse micronucleus assay
Экологическая судьба
Biological. When incubated with raw sewage and raw sewage acclimated with hydrocarbons,
benzyl chloride degraded forming nonchlorinated products (Jacobson and Alexander, 1981).
Chemical/Physical. Anticipated products from the reaction of benzyl chloride with ozone or OH
radicals in the atmosphere are chloromethyl phenols, benzaldehyde and chlorine radicals (Cupitt,
1980).
Slowly hydrolyzes in water forming HCl and benzyl alcohol. The estimated hydrolysis half-life
in water at 25 °C and pH 7 is 15 h (Mabey and Mill, 1978). The hydrolysis rate constant for
benzyl chloride at pH 7 and 59.2 °C was determined to be 0.0204/min, resulting in a half-life of 34
min (Ellington et al., 1986).
May polymerize in contact with metals except nickel and lead (NIOSH, 1997).
When heated to decomposition, hydrogen chloride gas may be released (CHRIS, 1984).
Перевозки
UN1738 Benzyl chloride, Hazard class: 6.1;
Labels: 6.1—Poisonous materials, 8—Corrosive material.
Методы очистки
Dry it with MgSO4 or CaSO4, or reflux it with fresh Ca turnings, then fractionally distil it under reduced pressure, collecting the middle fraction and storing it over CaH2 or P2O5. It has also been purified by passage through a column of alumina. Alternatively it is dried over MgSO4 and distilled in a vacuum. The middle fraction is degassed by several freeze-thaw cycles and then fractionated in an 'isolated fractionating column' (which has been evacuated and sealed off at ~10-6 mm) over a steam bath. The middle fraction is retained. The final samples are distilled in a vacuum from this sample and again retaining the middle fraction. The purity is >99.9% (no other peaks are visible by GLC, and the NMR spectrum is consistent with the structure. [Mohammed & Kosower J Am Chem Soc 93 1709 1971, Beilstein 5 IV 809.] IRRITANT and strongly LACHRYMATORY.
Несовместимости
May form explosive mixture with air.
Contact with water forms hydrogen chloride fumes. Strong
oxidizers may cause fire and explosions. Unstabilized benzyl
chloride undergoes polymerization with copper, aluminum,
iron, zinc, magnesium, tin, and other common metals
except lead and nickel, with the liberation of heat and
hydrogen chloride gas. May accumulate static electrical
charges, and may cause ignition of its vapors. Attacks some
plastics and rubber. Thermal decomposition and polymerization
reactions are inhibited, to a limited extent, by addition
of triethylamine, propylene oxide, or sodium carbonate.
Утилизация отходов
Incineration @ 816 C for
0.5 second minimum for primary combustion and 1204 C
for 12.0 second for secondary combustion. Elemental chlorine
formation may be alleviated by injection of steam or
methane into the combustion process.
Бензилхлорид препаратная продукция и сырье
сырьё
препарат