Хидамид химические свойства, назначение, производство
Описание
Chidamide (Epidaza®), a class I HDAC inhibitor, was discovered
and developed by ChipScreen and approved by the CFDA in
December 2014 for the treatment of recurrent of refractory peripheral
T-cell lymphoma. Chidamide, also known as CS055 and HBI-
8000, is an orally bioavailable benzamide type inhibitor of HDAC
isoenzymes class I 1–3, as well as class IIb 10, with potential antineoplastic
activity. It selectively binds to and inhibits HDAC, leading
to an increase in acetylation levels of histone protein H3.74 This
agent also inhibits the expression of signaling kinases in the PI3K/
Akt and MAPK/Ras pathways and may result in cell cycle arrest and
the induction of tumor cell apoptosis. Currently, phases I and II
clinical trials are underway for the treatment of non-small cell lung
cancer and for the treatment of breast cancer, respectively.
Использование
De-5-fluoro 4-Fluorochidamide is an analogue of Chidamide (CAS 743420-02-0), a hitsone deacetylase inhibitor (HDACI) that enhances gemcitabine (G305000) cytotoxicity in pancreatic cancer cells.
Синтез
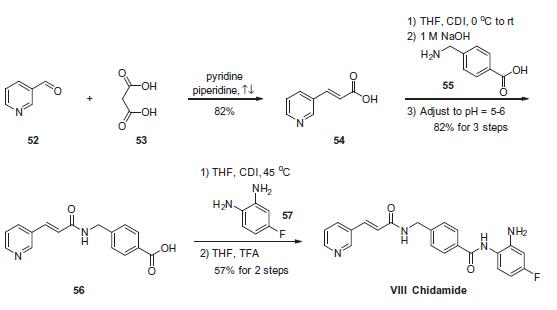
The scalable synthetic approach to chidamide very closely follows
the discovery route. The
sequence began with the condensation of commercial nicotinaldehyde
(52) and malonic acid (53) in a mixture of pyridine and
piperidine. Next, activation of acid 54 with N,N0-carbonyldiimidazole
(CDI) and subsequent reaction with 4-aminomethyl benzoic
acid (55) under basic conditions afforded amide 56 in 82% yield.
Finally, activation of 56 with CDI prior to treatment with 4-fluorobenzene-
1,2-diamine (57) and subsequent treatment with TFA
and THF yielded chidamide (VIII) in 38% overall yield from 52.
However, no publication reported that mono-N-Boc-protected
bis-aniline was used to approach Chidamide.
Хидамид препаратная продукция и сырье
сырьё
препарат