Лития гидрид химические свойства, назначение, производство
Химические свойства
Lithium hydride (LiH) is a crystalline salt substance(face-centered cubic) that is white in its pure form, As an engineering material, it has properties of interest in many technologies. For example,the high hydrogen content and light weight of LiH make it useful for neutron shields and moderators in nuclear power plants. In addition, the high heat of fusion combined with light weight make LiH appropriate for heat storage media for solar power plants on satellites and may be used as a heat sink for different applications. Typically, processes for production of LiH involve handling of LiH at temperatures above its meltingpoint (688 DC). Type 304L stainless steel is utilized for many process components handling molten LiH.
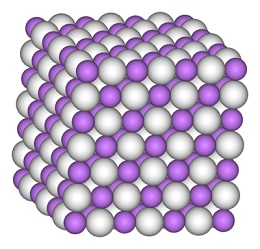
Lithium hydride is a typical ionic hydride with lithium cations and hydride anions. Electrolysis of molten material results in formation of lithium metal at the cathode and hydrogen at the anode. The lithium hydride-water reaction, which results in the release of hydrogen gas, is also indicative of a negatively charged hydrogen.
Физические свойства
White crystalline solid; cubic crystals; density 0.82 g/cm3; melts at 686.4°C; decomposes in water; soluble in acids.
Использование
Lithium hydride is used in the manufactureof lithium aluminum hydride and silane, as apowerful reducing agent, as a condensationagent in organic synthesis, as a portablesource of hydrogen, and as a lightweight nuclear shielding material. It is now beingused for storing thermal energy for spacepower systems (Morris et al. 1988).
Определение
lithium hydride: A white solid,LiH; cubic; r.d. 0.82; m.p. 680°C; decomposesat about 850°C. It is producedby direct combination of theelements at temperatures above500°C. The bonding in lithium hydrideis believed to be largely ionic;i.e. Li+H- as supported by the factthat hydrogen is released from theanode on electrolysis of the moltensalt. The compound reacts violentlyand exothermically with water toyield hydrogen and lithium hydroxide.It is used as a reducing agent toprepare other hydrides and the 2Hisotopic compound, lithiumdeuteride, is particularly valuable fordeuterating a range of organic compounds.Lithium hydride has alsobeen used as a shielding material forthermal neutrons.
Подготовка
Lithium hydride is prepared by heating lithium metal with hydrogen above 440°C. The reaction is exothermic and can be controlled once it is initiated, without external heating. The heat of formation is greater than that of sodium hydride:
2Li + H2 → 2LiH
Реакции
Lithium hydride reacts vigorously with water, forming lithium hydroxide with the evolution of hydrogen:
LiH + H2O → LiOH + H2
The hydride also reacts with ammonia forming lithium amide and evolving hydrogen:
LiH + NH3 → LiNH2 + H2
Lithium hydride is a strong reducing agent and would, therefore, react with compounds that contain oxygen. Even many highly stable oxides of metals and nonmetals can be reduced. It reduces metal oxides to metals and carbon dioxide to carbon:
Fe3O4 + 4LiH → 3Fe + 4NaOH
2LiH + CO2 → Li2O + C + H2O
It undergoes violent reactions with oxidizing agents.
Lithium hydride reacts with aluminum hydride forming lithium aluminum hydride, a powerful reducing agent:
LiH + AlH3 → LiAlH4
Lithium hydride consisting of Li+ and H– ions exhibits properties of an ionic salt, both cationic and anionic; such as a strong electrolyte. Thus, when electrolyzed at temperatures slightly below its melting point, it dissociates to Li+ and H¯ ions. Hydrogen gas is liberated at the anode.
The hydride ion, H:¯ being a strong base, would react with alcohols, forming alkoxides and liberating hydrogen:
CH3CH2OH + LiH → CH3CH2OLi + H2
(ethanol) (lithium ethoxide)
(CH3)3COH + LiH → (CH3)3COLi + H2
(tert-butanol) (lithium tert-butoxide)
Общее описание
A white or translucent crystalline mass or powder. The commercial product is light bluish-gray lumps due to the presence of minute amounts of colloidally dispersed lithium.
Реакции воздуха и воды
Burns readily in air, particularly if powdered. May ignite spontaneously in moist air. Reacts rapidly with water to form caustic lithium hydroxide and hydrogen [Bretherick 1979 p. 107].
Профиль реактивности
Lithium hydride is a strong reducing agent. May decompose violently in contact with most oxidizing materials. Reacts exothermically with water to form caustic lithium hydroxide and hydrogen gas; the hydrogen may ignite. May ignite spontaneously in moist air. Mixtures with liquid oxygen are explosive. Ignites on contact with dinitrogen oxide [Mellor, 1967, vol. 8, suppl. 2.2, p. 214].
Угроза здоровью
The health hazard due to lithium hydride maybe attributed to the following properties: (1)corrosivity of the hydride, (2) its hydrolysisto strongly basic lithium hydroxide, and (3)toxicity of the lithium metal. However, thelatter property, which may arise becauseof the formation of lithium resulting fromthe decomposition of lithium hydride andthe metabolic role of lithium, is not yetestablished.
This compound is highly corrosive to skin.Contact with eyes can produce severe irritationand possible injury. It can hydrolyzewith body fluid, forming lithium hydroxide,which is also corrosive to the skin andharmful to the eyes. Animal tests indicatedthat exposure to its dust or vapor at a levelexceeding 10 mg/m3 eroded the body fur andskin, caused severe inflammation of the eyes,and led to the destruction of external nasalseptum (ACGIH 1986). No chronic effectswere observed.
Пожароопасность
In a fire, irritating alkali fumes may form. Lithium hydride can form airborne dust clouds which may explode on contact with flame, heat, or oxidizing materials. Additionally, spontaneous ignition occurs when nitrous oxide and Lithium hydride are mixed. Lithium hydride also forms explosive mixtures with liquid oxygen. Contact with heat, moisture or acid causes exothermic reaction and evolution of hydrogen as well as lithium hydroxide. Incompatible with air and moisture, nitrous oxide, strong oxidizers, and liquid oxygen. Lithium hydride may ignite spontaneously in air and should be maintained and handled out of contact with air and moisture. Any contact with nitrous oxide; airborne powders may ignite upon reaching moisture.
Профиль безопасности
Poison by inhalation. A
severe eye, skin, and mucous membrane
irritant. Upon contact with moisture, lithium
hydroxide is formed. The LiOH formed is
very caustic and therefore highly toxic,
particularly to lungs and respiratory tract,
skin, and mucous membranes. The powder
ignttes spontaneously in air. The solid can
ignite spontaneously in moist air. Mixtures
of the powder with liquid oxygen are
explosive. Ignttes on contact with dinitrogen oxide, oxygen + moisture. To fight fire, use
special mixtures of dry chemical. See also
LITHIUM COMPOUNDS and
HYDRIDES.
Возможный контакт
Lithium hydride is used in preparation
of lithium aluminum hydride; as a desiccant; it is used in
hydrogen generators and in organic synthesis as a reducing
agent and condensing agent with ketones and acid esters; it
is reportedly used in thermonuclear weapons.
хранилище
The product should be handled under an inert atmosphere to avoid contamination and a fire. Powdered lithium hydride burns readily when exposed to the air. However, large pieces of the material are less flammable. Lithium hydride, like other strong bases, is harmful to the skin and should be handled with caution.
Перевозки
UN1414 Lithium, Hazard Class: 4.3; Labels:
4.3-Dangerous when wet material. UN2805 Lithium
hydride, fused solid, Hazard Class: 4.3; Labels: 4.3-
Dangerous when wet material
Методы очистки
It should be a white powder; otherwise replace it. It darkens rapidly on exposure to air and is decomposed by H2O to give H2 and LiOH, and reacts with lower alcohols. One gram in H2O liberates 2.8L of H2 (could be explosive). [D.nges in Handbook of Preparative Inorganic Chemistry (Ed. Brauer) Academic Press Vol I p 987 1963.]
Несовместимости
A Strong reducing agent. Incompatible
with oxidizers, halogenated hydrocarbons; acids can cause
fire and explosion. Reacts with water, forming caustic lithium hydroxide and flammable hydrogen gas; reaction may
cause ignition. May ignite spontaneously in moist air and
may reignite after fire is extinguished. Dangerous when
wet. Reacts with water to form hydrogen and lithium
hydroxide. Powdered form and liquid oxygen form an
explosive compound. Decomposes exothermically on contact with acids and upon heating to about 500�C, producing
flammable hydrogen gas. Reacts with carboxylic acids,
lower alcohols; chlorine, and ammonia (at 400�C), forming
explosive hydrogen gas.
Утилизация отходов
Lithium hydride may be
mixed with sand, sprayed with butanol and then with water,
neutralized and flushed to a sewer with water
Лития гидрид препаратная продукция и сырье
сырьё
препарат