
1,1,2-TRICHLORO-1,3,3,3-TETRAFLUOROPROPANE synthesis
- Product Name:1,1,2-TRICHLORO-1,3,3,3-TETRAFLUOROPROPANE
- CAS Number:812-30-6
- Molecular formula:C3Cl3F5
- Molecular Weight:237.38
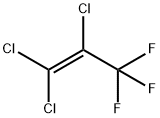
431-52-7
48 suppliers
$30.00/5G
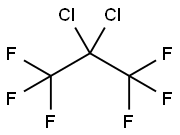
1652-80-8
10 suppliers
inquiry
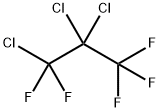
1599-41-3
28 suppliers
inquiry

812-30-6
10 suppliers
inquiry
Yield:1652-80-8 14.4 %Chromat. ,1599-41-3 63.4 %Chromat. ,812-30-6 8.5 %Chromat.
Reaction Conditions:
with hydrogen fluoride;chlorine;copper-substituted α-chromium oxide (99% chromium/1% copper) treated with HF at 280; under 760.051 Torr; for 0.00138889 h;Inert atmosphere;Monel nickel alloy tube packed with Monel turnings;
Steps:
8
General Procedure for Fluorination and ChlorofluorinationA weighed quantity of pelletized catalyst was placed in a inch (1.58 cm) diameter Inconel nickel alloy reactor tube heated in a fluidized sand bath: The tube was heated from 50° C. to 175° C. in a flow of nitrogen (50 cc/min; 8.3(10)-7 m3/sec) over the course of about one hour. HF was then admitted to the reactor at a flow rate of 50 cc/min (8.3(10)-7 m3/sec). After 0.5 to 2 hours the nitrogen flow was decreased to 20 cc/min (3.3(10)-7 m3/sec) and the HF flow increased to 80 cc/min (1.3(10)-6 m3/sec); this flow was maintained for about 1 hour. The reactor temperature was then gradually increased to 400° C. over 3 to 5 hours. At the end of this period, the HF flow was stopped and the reactor cooled to 300° C. under 20 sccm (3.3(10)-7 m3/sec) nitrogen flow. CFC-1213xa was fed from a pump to a vaporizer maintained at about 118° C. For fluorinations, the CFC-1213xa vapor was combined with the appropriate molar ratios of HF in a 0.5 inch (1.27 cm) diameter Monel nickel alloy tube packed with Monel turnings. The mixture of reactants then entered the reactor. The HF/1213xa molar ratio was 20 and the contact time was 5 seconds for Examples 1-7. For chlorofluorinations, the CFC-1213xa vapor was combined with the appropriate molar ratios of HF and chlorine. The HF/1213xa/chlorine molar ratio was 20/1/4 for all runs and the contact time was 5 seconds for Examples 8-14 and 30 seconds for Examples 15-16. The reactions were conducted at a nominal pressure of one atmosphere. Examples 8-16Chlorofluorination of 1213xaThe chlorofluorination of CFC-1213xa was carried out at various temperatures using catalysts prepared according to Catalyst Preparation Examples 1-9. The analytical results are shown in Table 2.Analytical data for identified compounds is given in units of GC area %. Small quantities of other unidentified products were present.
References:
US2010/152503,2010,A1 Location in patent:Page/Page column 12

431-52-7
48 suppliers
$30.00/5G
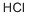
7647-01-0
11 suppliers
$10.00/10g

1652-80-8
10 suppliers
inquiry

1599-41-3
28 suppliers
inquiry

2804-50-4
23 suppliers
inquiry

431-87-8
22 suppliers
inquiry

76-18-6
26 suppliers
inquiry
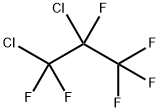
661-97-2
2 suppliers
inquiry

812-30-6
10 suppliers
inquiry

431-52-7
48 suppliers
$30.00/5G

812-30-6
10 suppliers
inquiry

431-52-7
48 suppliers
$30.00/5G
7647-01-0
11 suppliers
$10.00/10g

1652-80-8
10 suppliers
inquiry

1599-41-3
28 suppliers
inquiry

2804-50-4
23 suppliers
inquiry

431-87-8
22 suppliers
inquiry

76-18-6
26 suppliers
inquiry

661-97-2
2 suppliers
inquiry

812-30-6
10 suppliers
inquiry
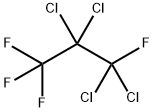
2268-44-2
4 suppliers
inquiry

431-52-7
48 suppliers
$30.00/5G

1652-80-8
10 suppliers
inquiry

1599-41-3
28 suppliers
inquiry

2804-50-4
23 suppliers
inquiry

431-87-8
22 suppliers
inquiry

76-18-6
26 suppliers
inquiry

661-97-2
2 suppliers
inquiry

812-30-6
10 suppliers
inquiry

2268-44-2
4 suppliers
inquiry