
1,1-Dichloroethane synthesis
- Product Name:1,1-Dichloroethane
- CAS Number:75-34-3
- Molecular formula:C2H4Cl2
- Molecular Weight:98.96
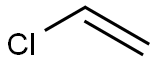
75-01-4
123 suppliers
$16.80/48625
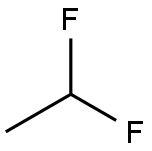
75-37-6
2 suppliers
$25.00/25 g

75-34-3
97 suppliers
$22.69/48602
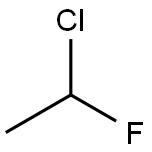
1615-75-4
1 suppliers
inquiry
Yield:-
Reaction Conditions:
with hydrogen fluoride;1,2-dichloro-ethane at 80; for 2 h;Conversion of starting material;
Steps:
6 Examples 6 to 12(In Accordance with the Invention)
A series of tests was performed at 80° C., in the same apparatus as that used in the above examples, starting from a liquid reaction mixture consisting of VC, HF, 1,2-dichloroethane as halogen-containing hydrocarbon and various hydrofluorination catalysts. The detailed operating conditions and the results are collated in Table I. These examples illustrate the influence of the nature of the catalyst on the quantitative distribution of the products obtained. In the absence of the catalyst or in the presence of vanadium tetrafluoride, chromium trifluoride or vanadium trichloride, the main product formed is 1-chloro-1-fluoroethane. In the presence of tin tetrachloride, molybdenum pentachloride or tungsten hexachloride, larger amounts of 1,1-difluorethane are formed. In all cases, the amount of heavy side products remains low. Depending on the catalyst used, the process thus allows a wide range of variation in the ratio between the 1-chloro-1-fluoroethane and 1,1-difluoroethane formed.
References:
Solvay (Societe Anonyme) US6809226, 2004, B1 Location in patent:Page column 7-8; Table I

75-01-4
123 suppliers
$16.80/48625

75-34-3
97 suppliers
$22.69/48602

75-01-4
123 suppliers
$16.80/48625

75-37-6
2 suppliers
$25.00/25 g

75-34-3
97 suppliers
$22.69/48602
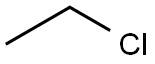
75-00-3
206 suppliers
$29.39/crm40015

75-34-3
97 suppliers
$22.69/48602

74-84-0
55 suppliers
$269.00/110 g

75-34-3
97 suppliers
$22.69/48602

75-01-4
123 suppliers
$16.80/48625

107-06-2
405 suppliers
$14.00/25g