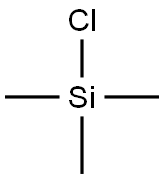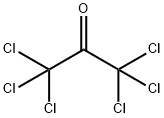
Hexachloroacetone synthesis
- Product Name:Hexachloroacetone
- CAS Number:116-16-5
- Molecular formula:C3Cl6O
- Molecular Weight:264.75

67-64-1
0 suppliers
$17.30/10ml

116-16-5
184 suppliers
$14.00/25G
Yield:116-16-5 98.4%
Reaction Conditions:
with chlorine;pyrographite at 150 - 160; for 7 h;Flow reactor;Industrial scale;Temperature;
Steps:
5
To a reactor made of nickel having an inner volume of 3 L equipped with a condenser was charged 16. 6 g of the same powdered activated carbon as in Example 1 which had been dried, followed by supplying chlorine molecule (B) at 0.3 L/min for 30 minutes. To the reactor was charged 1,499 g of hexachloroacetone (produced by Aldrich Co.) as a solvent, followed by initiating stirring, and raising the internal temperature to 150° C. The chlorine molecule (B) was supplied at 4 L/min, and the internal pressure was set to 0.3 MPaG. After 5 minutes from the initiation of the supply of chlorine molecule (B), acetone was supplied at 0.023 mol/min, and the heating was gradually performed so as to maintain the temperature from 150° C. to 160° C. At the time, a molar flow rate ratio of the chlorine molecule (B) and acetone was 7.7. After 6 hours from the initiation of the supply of acetone, the supply of acetone was terminated. At the time, as to the composition of the reaction liquid, hexachloroacetone was 97.2% by mass, pentachloroacetone was 2.0% by mass and other chloroacetone was 0.8% by mass. The flow rate of the chlorine molecule (B) was changed to 2 L/min and the reaction was further continued for one hour. In total, 448.0 g (7.71 mol) of the acetone and 4,779 g (67.4 mol) of the chlorine molecule (B) were supplied. The gas discharged from the reactor was passed through an absorption tower that was cooled to 0° C. and contained 629.1 g of hexachloroacetone as an absorption liquid, a cold trap at -20° C. and an about 20% by mass aqueous sodium hydroxide solution. In this manner, an organic substance, for example, hexachloroacetone or the compound (A) was recovered by the absorption tower and the cold trap, and the chlorine molecule (B) and hydrogen chloride were absorbed by the aqueous sodium hydroxide solution. After the completion of the reaction, the stirring was terminated while maintaining the temperature of the reaction mixture liquid to sediment the activated carbon, followed by taking out 1,703 g of the supernatant liquid through the insert tube. When the liquid taken out was analyzed by gas chromatography, it was found that 99.9% by mole was hexachloroacetone. At the time, it is estimated that 1,740 g of hexachloroacetone and 16.6 g of the activated carbon remain in the reactor. By using the remaining hexachloroacetone as the solvent and the remaining activated carbon as the catalyst, the chlorine molecule (B) and acetone were continuously added in the same manner as in the first time. After repeating the operation three times in total, the hexachloroacetone and activated carbon remaining in the reactor were also recovered. The acetone supplied in the four operations described above was 1,779 g (30.6 mol) in total, and the total of the supernatant liquid and the organic substance absorbed by the absorption liquid, which were recovered, was 9,566 g. From the analysis of the supernatant liquid and the absorption liquid recovered in each batch, it was found that the yield of hexachloroacetone was 98.4%, the yield of 1,1,1-trichloroacetone was 0.5% and the yield of other chloroacetones was 0.7% with respect to the acetone supplied, and the hexachloroacetone could be obtained in a very high yield. Also, the decrease in catalyst activity associated with the reuse of activated carbon was not found. The supernatant liquid recovered in each batch, and the hexachloroacetone and activated carbon remaining in the reactor were filtered through a polytetrafluoroethylene-made filter having a pore size of 0.2 μm to obtain a crude product. As a result of distilling the crude product, 1.0% by mass of a compound having a high boiling point with respect to the crude product was confirmed.
References:
ASAHI GLASS COMPANY, LIMITED;OKAMOTO, Hidekazu;TAJIMA, Kouhei;NAGANUMA, Tsutomu;FUJITA, Tomoyuki;NOMURA, Shingo US2016/304428, 2016, A1 Location in patent:Paragraph 0096-0098
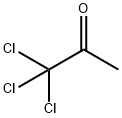
918-00-3
136 suppliers
$91.00/1g

116-16-5
184 suppliers
$14.00/25G
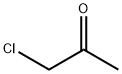
78-95-5
0 suppliers
$16.30/5 g

116-16-5
184 suppliers
$14.00/25G
![Silane, [[2,2-dichloro-1-(trichloromethyl)ethenyl]oxy]trimethyl-](/CAS/20200515/GIF/87651-34-1.gif)