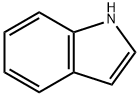
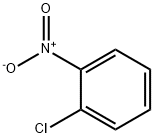
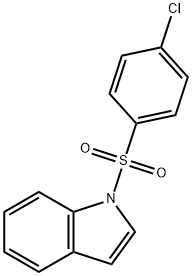
1-(2-Nitrophenyl)-1H-indole synthesis
- Product Name:1-(2-Nitrophenyl)-1H-indole
- CAS Number:25688-25-9
- Molecular formula:C14H10N2O2
- Molecular Weight:238.24
Yield:25688-25-9 93%
Reaction Conditions:
with tris(dibenzylideneacetone)dipalladium(0) chloroform complex;tri-tert-butyl phosphine;sodium t-butanolate in toluene at 90; for 12 h;
Steps:
5.1 First step: Preparation of compound Int-7
11.0 mmol of o-iodonitrobenzene and 10.0 mmol of indole, 1.2 g (12.0 mmol) of sodium tert-butoxide,An additional 52 mg (0.05 mmol) of Pd2(dba)3CHCl3 catalyst, 0.05 mL of 10% tri-tert-butylphosphine toluene solution and 60 mL of toluene were added.Under nitrogen protection, the temperature was raised to 90 ° C and the reaction was stirred for 12 hours, cooled to room temperature, and diluted with 60 mL of water.The mixture was extracted with EtOAc. EtOAc was evaporated.The compound Int-7 was obtained as a yellow solid in a yield of 93%.
References:
CN110156790,2019,A Location in patent:Paragraph 0150; 0152-0154