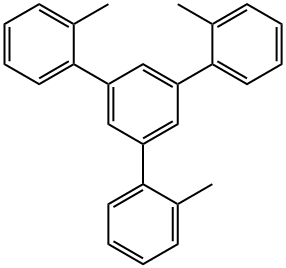
1,3,5-Tri-o-tolylbenzene synthesis
- Product Name:1,3,5-Tri-o-tolylbenzene
- CAS Number:87226-88-8
- Molecular formula:C27H24
- Molecular Weight:348.48
122382-54-1
0 suppliers
inquiry

87226-88-8
18 suppliers
$111.00/1g
Yield:87226-88-8 89%
Reaction Conditions:
with trifluorormethanesulfonic acid at 130; for 24 h;Inert atmosphere;
Steps:
2.1.1. 1,3,5-Tris-2-methylphenylbenzene (9)
2'-Methylacetophenone 8 (20.0 g, 150.0 mmol) and trifluoromethanesulfonicacid (2.50 g, 15.0 mmol) were added cautiouslyto a round-bottom flask before being stirred and heated at 130 °C under N2 for 24 h. During the course of the reaction a viscous blackmixture formed which solidified upon cooling to room temperature.The crude reaction mixture was quenched by addition ofwater (5 cm3) before being extracted with dichloromethane whichwas washed with water and brine, dried over MgSO4 and concentratedunder reduced pressure. The resulting crude product wasrecrystallised from chloroform and methanol giving 9 as a colourlesscrystalline solid (yield 16.0 g, 89%). 1H NMR (CD2Cl2, 400 MHz) δ 7.24-7.35 (m, 15H), 2.40 (s, 9H). 13C NMR (CDCl3, 400 MHz) δ 141.1, 140.9, 134.9, 130.0, 129.5, 128.1, 126.9, 125.4, 21.1. ESITOF-HRMS: m/z Calc. for C27H24: 348.1878. Found 348.1882 [M]+.Elemental Anal. Calc. for C27H24: C, 93.06; H, 6.94. Found: C,92.89; H, 6.87%. ATR-FTIR (cm-1): 3019 (w), 1423(m), 1389(w),1216(m), 1110(s), 754(s), 669(s).
References:
Argent, Stephen P.;Tarassova, Irina;Greenaway, Alex;Nowell, Harriot;Barnett, Sarah A.;Warren, Mark R.;Tang, Chiu C.;Morris, Christopher G.;Lewis, William;Champness, Neil R.;Schr?der, Martin;Blake, Alexander J. [Polyhedron,2016,vol. 120,p. 18 - 29]

626-39-1
478 suppliers
$5.00/5g
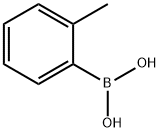
16419-60-6
377 suppliers
$9.00/1g

87226-88-8
18 suppliers
$111.00/1g
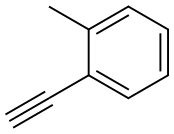
766-47-2
111 suppliers
$11.00/250mg
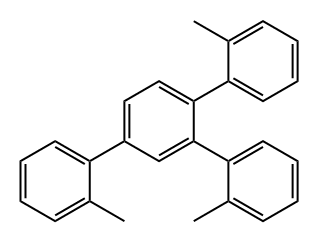
136612-95-8
0 suppliers
inquiry

87226-88-8
18 suppliers
$111.00/1g
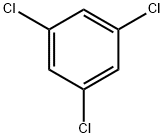
108-70-3
256 suppliers
$21.00/100g
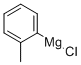
33872-80-9
85 suppliers
$99.29/100ml

87226-88-8
18 suppliers
$111.00/1g
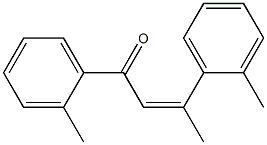
84118-71-8
0 suppliers
inquiry

87226-88-8
18 suppliers
$111.00/1g