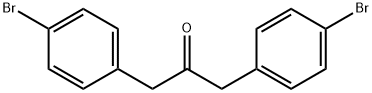
1,3-Bis(4-bromophenyl)propanone synthesis
- Product Name:1,3-Bis(4-bromophenyl)propanone
- CAS Number:54523-47-6
- Molecular formula:C15H12Br2O
- Molecular Weight:368.06
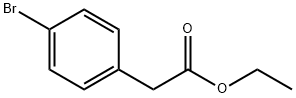
14062-25-0
235 suppliers
$6.00/5g

54523-47-6
75 suppliers
$31.00/200mg
Yield: 82%
Reaction Conditions:
with sodium hydride in toluene at 30 - 78;
Steps:
4.A A. SYNTHESIS OF 1,3-BIS(4-BROMOPHENYL)-2-PROPANONE
To a slurry of sodium hydride (9.17 grams, 0.23 mole) in 50 milliliters of toluene was added dropwise, a solution of ethyl 4-bromophenylacetate (50 grams, 0.21 mole) in toluene (50 milliliters) at 30-32°C. After addition was completed, the reaction mixture was slowly warmed to 50°C where the reaction began to rapidly exotherm with evolution of hydrogen gas. The reaction mixture was further heated to 78°C for 2 hours, cooled to room temperature and then HCl (45 grams) in water (22.5 grams) was slowly added dropwise to neutralize the solution. The layers were separated and the aqueous phase was extracted with diethylether. The combined organic extracts were dried and the solvent was removed to leave a yellow oil. This oil was refluxed for 24 hours in a mixture of glacial acid (60 milliliters) and concentrated HCl (30 milliliters). After cooling, the layers were separated, and the organic layer solidified to provide a yellow solid. This crude product was recrystallized from n-heptane to give a pure product as a white solid (31.2 grams, 82% isolated yield).
References:
Dow Global Technologies LLC;NIU, Qing, Shan, J.;HEFNER, Robert, E., Jr.;GODSCHALX, James, P.;ARNDT, Kim, E.;PECHACEK, James T. EP1476416, 2015, B1 Location in patent:Paragraph 0058
1878-68-8
484 suppliers
$6.00/10g

54523-47-6
75 suppliers
$31.00/200mg
37859-24-8
94 suppliers
$26.10/1g
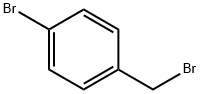
589-15-1
438 suppliers
$6.00/10g

54523-47-6
75 suppliers
$31.00/200mg
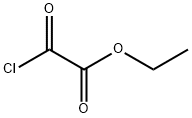
4755-77-5
334 suppliers
$9.00/25g

589-15-1
438 suppliers
$6.00/10g

54523-47-6
75 suppliers
$31.00/200mg
201230-82-2
1 suppliers
inquiry

589-15-1
438 suppliers
$6.00/10g
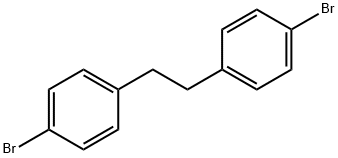
19829-56-2
19 suppliers
$60.00/250 mg

54523-47-6
75 suppliers
$31.00/200mg
![Ethanamine, N-[(3,5-dichlorophenyl)methylene]-2,2-diethoxy-](/CAS/20210305/GIF/1000210-73-0.gif)
1000210-73-0
0 suppliers
inquiry

1878-68-8
484 suppliers
$6.00/10g