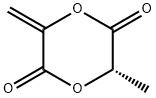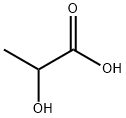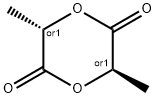
1,4-Dioxane-2,5-dione,3,6-dimethyl-, (3R,6S)- synthesis
- Product Name:1,4-Dioxane-2,5-dione,3,6-dimethyl-, (3R,6S)-
- CAS Number:13076-19-2
- Molecular formula:C6H8O4
- Molecular Weight:144.13
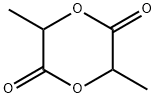
95-96-5
260 suppliers
$7.00/1g

13076-19-2
15 suppliers
inquiry
Yield:13076-19-2 88.2%
Reaction Conditions:
with 1,4-diaza-bicyclo[2.2.2]octane;tris(pentafluorophenyl)borate in toluene at 20; for 10 h;
Steps:
4 Procedure I for Isolation of the Pure Rac-EA
Epimerization of meso-EA was performed in a glovebox. To a solution of meso-EA (5.76 g, 40 mmol) in anhydrous toluene (19.2 mE) was added B(C5F5)3 (0.4 mE, 0.002 M in toluene) and DAI3CO (0.4 mE, 0.002 M in toluene). The mixture was stirred at room temperature for 10 h, afier which time the resulting suspension was filtered and washed with cold toluene (15 mE) twice, and dried under vacuum at room temperature to give the pure rac-EA (run 38, Table A and FIG. 4) as a white solid (5.08 g, yield:88.2%). The filtrate was a mixture of rac-EA and meso-EAin a 95:5 ratio.
References:
US2018/244841,2018,A1 Location in patent:Paragraph 0096
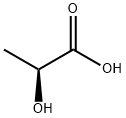
79-33-4
519 suppliers
$6.00/25g

13076-19-2
15 suppliers
inquiry

4511-42-6
277 suppliers
$16.80/5G
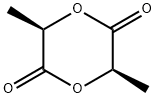
13076-17-0
129 suppliers
$6.00/1g