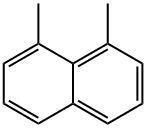
1,8-DIMETHYLNAPHTHALENE synthesis
- Product Name:1,8-DIMETHYLNAPHTHALENE
- CAS Number:569-41-5
- Molecular formula:C12H12
- Molecular Weight:156.22
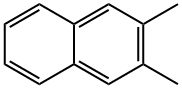
581-40-8
127 suppliers
$41.60/1g
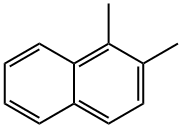
573-98-8
88 suppliers
$22.60/250mg
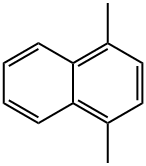
571-58-4
102 suppliers
$70.70/100mg
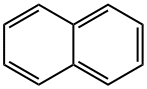
91-20-3
490 suppliers
$13.19/250mg
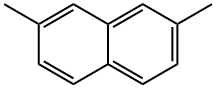
582-16-1
165 suppliers
$53.00/250mg
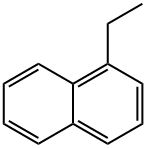
1127-76-0
135 suppliers
$6.00/1g
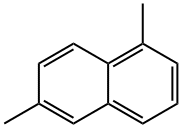
575-43-9
76 suppliers
$75.00/500 mg
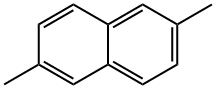
581-42-0
158 suppliers
$50.00/1 g
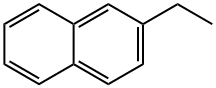
939-27-5
88 suppliers
$16.00/1g
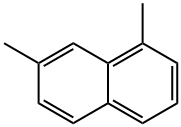
575-37-1
58 suppliers
$60.00/5 mg

575-41-7
91 suppliers
$85.00/1g

569-41-5
75 suppliers
$45.00/10mg
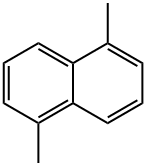
571-61-9
84 suppliers
$34.00/100mg
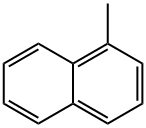
90-12-0
268 suppliers
$17.00/25mL
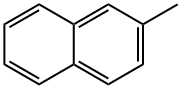
91-57-6
242 suppliers
$13.00/5g
Yield:91-20-3 3.11 %Chromat. ,91-57-6 15.68 %Chromat. ,90-12-0 3.71 %Chromat. ,939-27-5 0.57 %Chromat. ,1127-76-0 0.26 %Chromat. ,581-42-0 4.86 %Chromat. ,582-16-1 4.58 %Chromat. ,575-43-9 2.92 %Chromat. ,571-61-9 0.39 %Chromat. ,569-41-5 0.12 %Chromat.
Reaction Conditions:
with hydrogen;chromium corundum at 20 - 475; under 44929.5 Torr;Conversion of starting material;
Steps:
4 Example 4 (Hydrodealkylation)
Example 4 (Hydrodealkylation) A part of Fraction-17 and Residue shown in Table 3 are mixed to prepare the feedstock(Blend-A) for hydrodealkylation. A 50 g amount of Cr2O3/Al2O3 type catalyst produced by Sud-Chemie AG is charged into a tubular reactor. The reactor is heated gradually from ambient temperature to 662F (350 °C) to dry the catalyst while supplying hydrogen gas. Thereupon Blend-A is fed to the reactor at the rate of 50 g/hr and 1.0 hr-1 in WHSV, while supplying hydrogen gas at 1.25 cf/hr (0.034 m3/hr). Hydrodealkylation is carried out at 887F (475 °C) and 854 psig (5.99 MPa). The product is analyzed by GC and the results of hydrodealkylation are summarized in Table 4 below. As shown in Table 4, Cr2O3/Al2O3 type catalyst is effective to enrich 2,6-DMN from 2,6-DMN lean feed.
References:
KABUSHIKI KAISHA KOBE SEIKO SHO;EXXONMOBIL OIL CORPORATION EP1165473, 2004, B1 Location in patent:Page 9-10
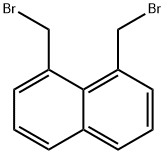
2025-95-8
37 suppliers
$47.60/250mg

569-41-5
75 suppliers
$45.00/10mg

81-84-5
220 suppliers
$13.00/25g

569-41-5
75 suppliers
$45.00/10mg
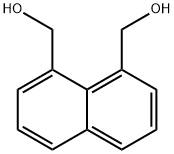
2026-08-6
40 suppliers
$45.00/10mg

569-41-5
75 suppliers
$45.00/10mg
![1H,3H-Naphtho[1,8-cd]pyran](/CAS/GIF/203-84-9.gif)
203-84-9
0 suppliers
inquiry

569-41-5
75 suppliers
$45.00/10mg
10336-29-5
24 suppliers
$243.00/250mg