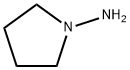
1-AMINOPYRROLIDINE synthesis
- Product Name:1-AMINOPYRROLIDINE
- CAS Number:16596-41-1
- Molecular formula:C4H10N2
- Molecular Weight:86.14
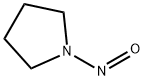
930-55-2
113 suppliers
$55.00/1g

16596-41-1
86 suppliers
$65.00/100mg
Yield:16596-41-1 92%
Reaction Conditions:
with carbon dioxide;water;ammonium chloride;zinc at 35; under 1500.15 Torr; for 1.5 h;Autoclave;Green chemistry;
Steps:
Synthesis of N-amino aza-aliphatic cyclic compounds 2a-c, 5a,b, 7a,b, and 9
General procedure: A solution ofN-nitroso aza-aliphatic cyclic compound 1a-c, 4a,b, 6a,b, or 8 (1.0 mol) in H2O (250 ml) was placed into a 1-l autoclave equipped with a CO2 ventilation device, a gas flow meter, and a barometer. NH4Cl (5.35 g, 0.1 mol) was added to the solution and the system was charged with CO2 at a flow rate 30 l/h. After stirring for 20 min, the mixture was heated to 35°C and zinc powder (163 g, 2.5 mol) was added in portions (16.3 g every 6 min). The pressure in the closed system was maintained at 0.2 MPa and temperature at 35°C. After addition of all zinc (about 1 h), the reaction mixture was stirred at 35°C for 0.5 h and then analyzed by GC. The resulting ZnCO3 was filtered off and washed with H2O (150 ml). The filtrate was transferred to a 1-l three neck round-bottom flask, H2O was removed under reduced pressure, and vacuum distillation at 60-135 °C (internal temperature) was performed to afford products 2a-c, 5a,b, 7a,b, and 9.
References:
Yang, Weiqing;Lu, Xiang;Zhou, Tingting;Cao, Yongjing;Zhang, Yuanyuan;Ma, Menglin [Chemistry of Heterocyclic Compounds,2018,vol. 54,# 8,p. 780 - 783][Khim. Geterotsikl. Soedin.,2018,vol. 54,# 8,p. 780 - 783,4]

110-56-5
317 suppliers
$14.00/25mL

16596-41-1
86 suppliers
$65.00/100mg

110-52-1
577 suppliers
$9.00/1g

7803-57-8
0 suppliers
$20.10/50g

16596-41-1
86 suppliers
$65.00/100mg

930-55-2
113 suppliers
$55.00/1g
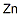
7440-66-6
0 suppliers
$14.00/5g

16596-41-1
86 suppliers
$65.00/100mg

110-52-1
577 suppliers
$9.00/1g

16596-41-1
86 suppliers
$65.00/100mg