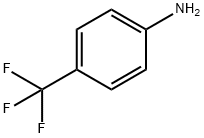
1-BOC-4-(4-TRIFLUOROMETHYL-PHENYLAMINO)-PIPERIDINE synthesis
- Product Name:1-BOC-4-(4-TRIFLUOROMETHYL-PHENYLAMINO)-PIPERIDINE
- CAS Number:401565-92-2
- Molecular formula:C17H23F3N2O2
- Molecular Weight:344.37
Yield:401565-92-2 62.4%
Reaction Conditions:
with borane-THF;glacial acetic acid in dichloromethane at 0 - 25; for 12 h;Inert atmosphere;
Steps:
a Step a) tert-Butyl 4-[4-(trijluoromethyl)anilinoJpiperidine- 1-carboxylate
To a solution of p-trifluoromethylaniline (1.17 mL, 9.31 mmol, CAS RN 455-14-1) in DCM (30 mL) was added AcOH (0.560 g, 9.31 mmol) and 1-BOC-4-piperidone (2.78 g, 14.0 mmol, CAS RN 79099-07-3). Then 1M BH3/THF solution (27.9 mL, 27.9 mmol) was addedcarefully at 0 °C under nitrogen atmosphere. The reaction mixture was stirred at 25 °C for 12 h. The mixture was poured into saturated aqueous NH4C1 solution (30 mL) and extracted three times with EtOAc. The combined organic layers were washed twice with water H20, and then brine, dried over Na2504 and concentrated in vacuum to afford yellow residue, which was purified by silica gel column eluting with a gradient of PE : EtOAc (20: ito 5 : 1) to give thedesired product as white solid (2.0 g, 62.4%). MS (ESI): m/z = 289.1 [M-56+H].
References:
WO2020/35425,2020,A1 Location in patent:Page/Page column 196
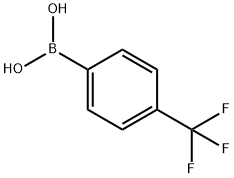
128796-39-4
425 suppliers
$10.00/5g
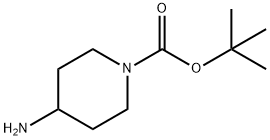
87120-72-7
20 suppliers
$6.00/1g

401565-92-2
13 suppliers
inquiry