
1-BROMO-8-METHYLNAPHTHALENE synthesis
- Product Name:1-BROMO-8-METHYLNAPHTHALENE
- CAS Number:33295-37-3
- Molecular formula:C11H9Br
- Molecular Weight:221.09
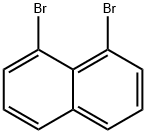
17135-74-9
271 suppliers
$8.00/250mg
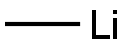
917-54-4
173 suppliers
$44.39/50ml

74-88-4
342 suppliers
$15.00/10g

33295-37-3
55 suppliers
inquiry
Yield: 43%
Reaction Conditions:
Stage #1:1,8-dibromonaphthalene;methyllithium in tetrahydrofuran;diethyl ether at 0; for 0.5 h;
Stage #2:methyl iodide in tetrahydrofuran;diethyl ether at 0 - 25; for 3 h;
Steps:
[0304] Step A: 1 -bromo-8-methyl-naphthalene.
To a solution of 1,8-dibromonaphthalene (1 g, 3.50 mmol, 1 eq) in THF (20 L) was added MeLi (1.6 M in diethyl ether, 2.62 mL, 1.2 eq) at 0°C dropwise. After stirring for 30 minutes at 0°C, iodomethane (3.38 g, 23.8 mmol, 1.48 mL, 6.81 eq) was added dropwise. The mixture was warmed up to 25°C and stirred for another 3 hours. The reaction mixture was quenched with water (20 mL) and extracted with ethyl acetate (20 mL x 3). The combined organic layers were washed with brine (20 mL), dried over Na2S04, filtered and concentrated under reduced pressure to give a residue. The residue was purified by prep-HPLC (column: Phenomenex Gemini C l 8 250*50mm* 10 um; mobile phase: [water (0.05% ammonia hydroxide v/v) - ACN]; B%: 45% - 70%, 28 MiN; 40% min). Title compound l-bromo-8-methyl- naphthalene (340 mg, 1.49 mmol, 43% yield, 97% purity) was obtained as a yellow solid after lyophilisation. [0305] .H NMR (400MHZ, ch.oroform-d) d - 7.75 (dd,.7 - 0.8, 7.2 Hz, 1H), 7.69 (dd, J= 0.8, 8.0 Hz, 1H), 7.66 - 7.59 (m, 1H), 7.30 - 7.22 (m, 2H), 7.13 (t, J= 8.0 Hz, 1H), 3.05 (s, 3H).
References:
MIRATI THERAPEUTICS, INC.;ARRAY BIOPHARMA, INC.;MARX, Matthew, Arnold;CHRISTENSEN, James, Gail;SMITH, Christopher, Randolph;FISCHER, John, P.;BURNS, Aaron, Craig WO2020/146613, 2020, A1 Location in patent:Paragraph 0304-0305

17135-74-9
271 suppliers
$8.00/250mg

74-88-4
342 suppliers
$15.00/10g

33295-37-3
55 suppliers
inquiry
14938-58-0
50 suppliers
$98.00/50mg

33295-37-3
55 suppliers
inquiry
4044-58-0
79 suppliers
$60.00/10 mg

33295-37-3
55 suppliers
inquiry
62456-34-2
75 suppliers
inquiry

33295-37-3
55 suppliers
inquiry