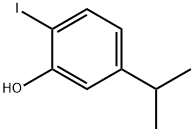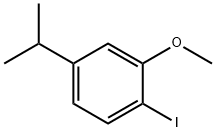
1-Iodo-4-isopropyl-2-methoxybenzene synthesis
- Product Name:1-Iodo-4-isopropyl-2-methoxybenzene
- CAS Number:1240304-64-6
- Molecular formula:C10H13IO
- Molecular Weight:276.11
Yield:1240304-64-6 85%
Reaction Conditions:
with potassium carbonate in acetonitrile at 40; for 3.5 h;
Steps:
1.2 Reference Example 1-2 Compound (B2)
Reference Example 1-2 Compound (B2) [0121] [0122] Methyl iodide (9.8 mL, 0.156 mol) was added to an acetonitrile suspension (200 mL) of the compound (B1) (27.4 g, 0.104 mol) and potassium carbonate (21.7 g, 0.156 mol), and the mixture was stirred for 2.5 hours at 40° C. Methyl iodide (3.5 mL, 0.052 mol) was further added, and the mixture was stirred for 1 hour at the same temperature. Insolubles were filtered out, and the filtrate was diluted with ethyl acetate. The organic layer was washed with water, a 10% aqueous solution of sodium thiosulfate, and brine, and dried over anhydrous magnesium sulfate. The desiccant was filtered out, and then the solvent was distilled off under reduced pressure. The resulting residue was purified by neutral silica gel column chromatography (hexane→hexane:ethyl acetate=95:5) to obtain a light yellow oily compound (B2) (24.5 g, 85%). [0123] 1H NMR (300 MHz, CHLOROFORM-d) δ ppm 1.24 (d, J=6.84 Hz, 6H) 2.87 (quin, J=6.92 Hz, 1H) 3.88 (s, 3H) 6.58-6.65 (m, 1H) 6.70 (d, J=1.87 Hz, 1H) 7.65 (d, J=8.08 Hz, 1H). MS ESI/APCI Dual posi: 277[M+H]+.
References:
US2013/144050,2013,A1 Location in patent:Paragraph 0121; 0122; 0123

1202161-10-1
1 suppliers
inquiry

1240304-64-6
19 suppliers
inquiry
618-45-1
97 suppliers
$67.00/5g

1240304-64-6
19 suppliers
inquiry

55311-42-7
8 suppliers
inquiry

1240304-64-6
19 suppliers
inquiry

6380-20-7
12 suppliers
$215.00/5G

1240304-64-6
19 suppliers
inquiry