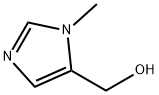
(1-METHYL-1H-IMIDAZOL-5-YL)METHANOL synthesis
- Product Name:(1-METHYL-1H-IMIDAZOL-5-YL)METHANOL
- CAS Number:38993-84-9
- Molecular formula:C5H8N2O
- Molecular Weight:112.13
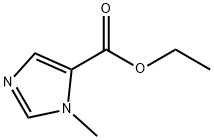
66787-70-0
53 suppliers
$45.00/100mg

38993-84-9
115 suppliers
$18.00/250mg
Yield: 73%
Reaction Conditions:
with lithium aluminium tetrahydride in tetrahydrofuran at 20; for 16 h;
Steps:
8
S-Methyl-SH-imidazole^-carboxylic acid ethyl ester (Chem. Pharm. Bull, 1994, 42,1463) (0.62 g, 4.02 mmol) in THF (5mL) was added dropwise over 1 min. to a slurry of lithium aluminum hydride (0.25 g, 6.60 mmol) in THF (20 mL). After stirring 16h at room temperature, the reaction was carefully quenched with excess sodium sulfate decahydrate, dried with anhydrous sodium sulfate and filtered through Celite. Concentration gave 0.33 g (73%) of (3- methyl-3H-imidazol-4-yl)-methanol as a white solid which had: NMR (CDCI3) δ 7.38 (s, 1 H), 6.87 (s, 1 H), 4.60 (s, 2H)1 3.69 (s, 3H). A solution of this alcohol (0.150 g, 1.34 mmol) in thionyl chloride (5 mL) was refluxed for 3 h and concentrated. The residue was dissolved in a minimum of ethanol and ether was added to precipitate a white solid. This was collected to yield 0.185 g (83%) of 5-chloromethyl-1-methyl-1H-imidazole hydrochloride which had: NMR (DMSOd6) δ 9.15 (s, 1 H), 7.75 (d, J = 1.2 Hz, 1 H), 4.99 (s, 2H), 3.84 (s, 3H).
References:
PFIZER PRODUCTS INC. WO2007/34277, 2007, A1 Location in patent:Page/Page column 58
39021-62-0
199 suppliers
$35.00/1g

38993-84-9
115 suppliers
$18.00/250mg
143122-18-3
44 suppliers
$75.00/250mg

38993-84-9
115 suppliers
$18.00/250mg
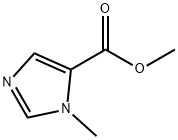
17289-20-2
144 suppliers
$9.00/250mg

38993-84-9
115 suppliers
$18.00/250mg
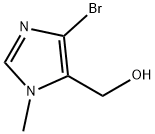
141524-73-4
11 suppliers
$45.00/2.5mg

38993-84-9
115 suppliers
$18.00/250mg