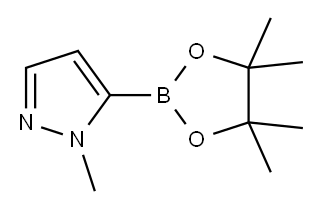
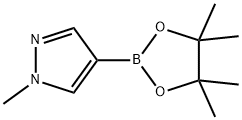
1-Methyl-1H-pyrazole-5-boronic acid pinacol ester synthesis
- Product Name:1-Methyl-1H-pyrazole-5-boronic acid pinacol ester
- CAS Number:847818-74-0
- Molecular formula:C10H17BN2O2
- Molecular Weight:208.07
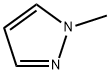
930-36-9
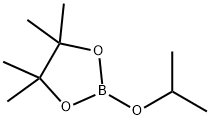
61676-62-8

847818-74-0
To a solution of 1-methylpyrazole (4.1 g, 50 mmol) in tetrahydrofuran (THF, 100 mL) was slowly added n-butyllithium (n-BuLi, 2.2 M solution in THF, 55 mmol) at 0 °C. The reaction mixture was stirred at room temperature for 1 hour and then cooled to -78 °C. Subsequently, 2-isopropoxy-4,4,5,5-tetramethyl-1,3,2-dioxaborolane (12.3 mL, 60 mmol) was added to the reaction system. The reaction was carried out at -78 °C for 15 min and then gradually warmed up to 0 °C, taking 1 hour. Upon completion of the reaction, the reaction was quenched with saturated ammonium chloride (NH4Cl) solution and extracted with dichloromethane (DCM). The organic phases were combined, washed with distilled water (2 × 100 mL), dried over anhydrous sodium sulfate (Na2SO4), and concentrated under reduced pressure to afford the brown solid product 1-methyl-1H-pyrazole-5-boronic acid pinacol ester (8.0 g, 77% yield), which could be used for the subsequent reaction without further purification. The product was detected by liquid chromatography-mass spectrometry (LCMS, ES), showing m/z 127 (M+H)+ [RB(OH)2]; nuclear magnetic resonance hydrogen spectroscopy (1H NMR, CDCl3, 400 MHz) showed δ 7.57 (s, 1H), 6.75 (s, 1H), 4.16 (s, 3H) and 1.41 (s, 12H).

930-36-9
341 suppliers
$5.00/1g

61676-62-8
323 suppliers
$10.00/5g

847818-74-0
265 suppliers
$6.00/1g
Yield:847818-74-0 77%
Reaction Conditions:
Stage #1:1-methyl-1H-pyrazole with n-butyllithium in tetrahydrofuran at 0 - 20; for 1 h;
Stage #2:2-Isopropoxy-4,4,5,5-tetramethyl-1,3,2-dioxaborolane in tetrahydrofuran at -78 - 0; for 1.25 h;
Stage #3: with ammonium chloride in tetrahydrofuran
Steps:
2
To a solution of 1-methyl pyrazole (4.1 g, 50 mmole) in THF (100 mL) at 00C was added n-BuLi (2.2M in THF, 55 mmole). The reaction solution was stirred for 1 hour at RT and then cooled to -78°C [J. Heterocyclic Chem. 41 , 931 (2004)]. To the reaction solution was added 2-isopropoxy-4,4,5,5-tetramethyl-1 ,3,2-dioxaborolane (12.3 mL, 60 mmole). After 15 min at -78°C, the reaction was allowed to warm to 00C over 1 hour. The reaction
References:
SMITHKLINE BEECHAM CORPORATION WO2009/158372, 2009, A1 Location in patent:Page/Page column 30; 31

5419-55-6
388 suppliers
$12.00/5g

847818-74-0
265 suppliers
$6.00/1g
76-09-5
397 suppliers
$8.19/250mg
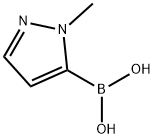
720702-41-0
177 suppliers
$12.00/250mg

847818-74-0
265 suppliers
$6.00/1g

930-36-9
341 suppliers
$5.00/1g
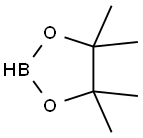
25015-63-8
216 suppliers
$22.39/5G

847818-74-0
265 suppliers
$6.00/1g

930-36-9
341 suppliers
$5.00/1g

25015-63-8
216 suppliers
$22.39/5G

847818-74-0
265 suppliers
$6.00/1g
761446-44-0
374 suppliers
$10.00/1g