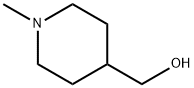
1-Methyl-4-piperidinemethanol synthesis
- Product Name:1-Methyl-4-piperidinemethanol
- CAS Number:20691-89-8
- Molecular formula:C7H15NO
- Molecular Weight:129.2
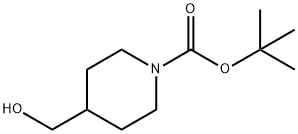
123855-51-6
420 suppliers
$6.00/5g

20691-89-8
243 suppliers
$6.00/1g
Yield:20691-89-8 88%
Reaction Conditions:
Stage #1: tert-butyl 4-(hydroxymethyl)piperidin-1-carboxylatewith lithium aluminium tetrahydride in tetrahydrofuran at 20;Inert atmosphere;
Stage #2: with water;sodium hydroxide in tetrahydrofuran at 0 - 20;
Steps:
1 (1-Methylpiperidin-4-yl)methyl 4-nitrophenyl carbonate
A solution of tert-butyl 4-(hydroxymethyl)piperidine-1-carboxylate (1.94 g, 9.0 mmol) in THF (15.0 mL) was added drop-wise to a 1M solution of LiAlH4 in THF (13.5 mL, 13.5 mmol) under argon. The reaction mixture was stirred at room temperature for 17 hours and then cooled to 0° C. A mixture of THF and water (1:1 ratio, 1.5 mL) was added drop-wise. A gelatinous white solid formed. 4M aq NaOH solution (0.6 mL) was added drop-wise. Water (2 mL) was added and the resulting mixture stirred at room temperature for 2 hours. The white solid was removed by filtration. The filtrate was loaded onto an Isolute HM-N liquid-liquid extraction column and then eluted with EtOAc (200 mL). The resulting organic phase concentrated in vacuo yielding (1-methylpiperidin-4-yl)methanol as a yellow oil (1.02 g, 88%). Analytical LCMS: purity ~90% (System B, RT=1.88 min), ES+: 129.8 [MH]+. (
References:
US2009/281087,2009,A1 Location in patent:Page/Page column 9-10

24252-37-7
189 suppliers
$6.00/1g

20691-89-8
243 suppliers
$6.00/1g

1690-75-1
163 suppliers
$8.00/5g

20691-89-8
243 suppliers
$6.00/1g
6457-49-4
389 suppliers
$18.00/25g

20691-89-8
243 suppliers
$6.00/1g
36166-75-3
26 suppliers
$130.00/50mg

20691-89-8
243 suppliers
$6.00/1g