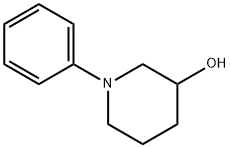
1-Phenylpiperidin-3-ol synthesis
- Product Name:1-Phenylpiperidin-3-ol
- CAS Number:80710-25-4
- Molecular formula:C11H15NO
- Molecular Weight:177.24
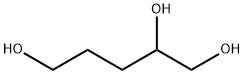
14697-46-2
57 suppliers
$45.00/50mg

62-53-3
637 suppliers
$10.00/1g

80710-25-4
44 suppliers
$130.00/50mg

18133-14-7
0 suppliers
inquiry
Yield:18133-14-7 26% ,80710-25-4 28%
Reaction Conditions:
Stage #1: 1,2,5-pentanetriolwith [ruthenium(II)(η6-1-methyl-4-isopropyl-benzene)(chloride)(μ-chloride)]2;caesium carbonate;4,5-bis(diphenylphosphino)-9,9-dimethylxanthene in toluene at 20; for 0.666667 h;Molecular sieve;Inert atmosphere;Schlenk technique;Green chemistry;
Stage #2: aniline in toluene at 20; for 48.33 h;Inert atmosphere;Schlenk technique;Sealed tube;Reflux;Green chemistry;
Steps:
Typical synthetic procedure (with 4a as an example)
General procedure: Xantphos (28.9 mg, 0.05 mmol), Cs2CO3 (32.6 mg, 0.10 mmol) and [Ru(p-cymene)Cl2]2 (15.3 mg, 0.025 mmol) were added to a Schlenk tube. Then the tube was evacuated and refilled with nitrogen. Dry toluene as solvent (2.0 mL)were added and the solution was stirred at room temperature under nitrogen for 30 min. Activated molecular sieves 4? (0.10 g) and triol 6a (106.1 mg, 1.00 mmol) were then added. And the solution was stirred at room temperature under nitrogen for 40 min. Subsequently, aniline 5a (46.6 mg, 0.50 mmol) was added and the solution was stirred at room temperature under nitrogen for 20 min. Then the resulting solution was heated to reflux and stirred for 48 h. The reaction was monitored by TLC. After completion, the solution was filtered through a plug of Celite and washed with EtOAc (3 mL x 3). The filtrate was concentrated under vacuum. Purification via flash column chromatography with silica gel (eluting with PE/EA = 5/1 (v/v)) yielded 4a (43.0 mg, 81% yield) as light yellow oil.
References:
Xu, Qing-Song;Li, Chen;Xu, Yong;Xu, Defeng;Shen, Mei-Hua;Xu, Hua-Dong [Chinese Chemical Letters,2020,vol. 31,# 1,p. 103 - 106] Location in patent:supporting information

15941-41-0
2 suppliers
inquiry

80710-25-4
44 suppliers
$130.00/50mg
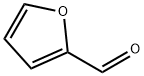
98-01-1
481 suppliers
$24.30/250g

80710-25-4
44 suppliers
$130.00/50mg

62-53-3
637 suppliers
$10.00/1g

80710-25-4
44 suppliers
$130.00/50mg