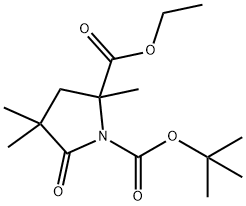
ETHYL N-BOC-2,4,4-TRIMETHYL-5-OXOPYRROLIDINE-2-CARBOXYLATE synthesis
- Product Name:ETHYL N-BOC-2,4,4-TRIMETHYL-5-OXOPYRROLIDINE-2-CARBOXYLATE
- CAS Number:1001353-88-3
- Molecular formula:C15H25NO5
- Molecular Weight:299.36
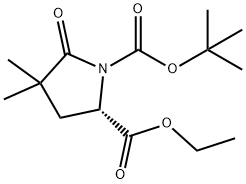
158392-80-4
24 suppliers
$339.63/1g

74-88-4
340 suppliers
$15.00/10g

1001353-88-3
14 suppliers
inquiry
Yield:1001353-88-3 72%
Reaction Conditions:
with lithium hexamethyldisilazane in tetrahydrofuran at -78 - 20; for 4 h;
Steps:
163.E
163E. 1-tert-Butyl 2-ethyl 2,4,4-trimethylpyrrolidine-1,2-dicarboxylate To a solution of(s)-1-tert-butyl 2-ethyl 4,4-dimethyl-5-oxopyrrolidine-1,2-dicarboxylate (163D) (10 g, 0.035 mol) in dry THF (60 mL) stirred at -78° C. under nitrogen was added a 1 M solution of lithium hexamethyldisilazide in THF (38.5 mL, 0.038 mol). The mixture was stirred at -78° C. for 30 min, iodomethane (5.4 g, 0.038 mol) in THF (5 mL) was added, and the mixture was then stirred 1 h, warmed up to room temperature and continued stirring for additional 3 h. The reaction mixture was quenched with saturated ammonium chloride solution and extracted with CH2Cl2 (3*100 mL). The combined organic layers were dried over Na2SO4, filtered, and evaporated to dryness. The residue was purified by flash chromatography (10-20% EtOAc/hexane) to furnish (163E) (8.2 g, 72%). 1H NMR (CDCl3, 400 MHz)) δ 4.17 (2H, q, J=7.2 Hz), 2.12 (1H, d, J=13.6 Hz), 1.84 (1H, d, J=13.6 Hz), 1.68 (3H, s), 1.48 (9H, s), 1.24 (3H, t, J=7.2 Hz), 1.25 (3H, s), and 1.22 (3H, s). 13C NMR (CDCl3, 100 MHz) δ 179.00, 173.41, 149.65, 83.51, 62.61, 61.61, 45.62, 40.96, 27.95 (3C), 27.51, 26.75, 25.06, and 14.06.
References:
US2008/9497,2008,A1 Location in patent:Page/Page column 71

24424-99-5
824 suppliers
$13.50/25G

1001353-88-3
14 suppliers
inquiry
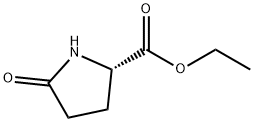
7149-65-7
229 suppliers
$15.00/25G

1001353-88-3
14 suppliers
inquiry