
4,4,5,5-tetramethyl-2-(thieno[3,2-b]thiophen-2-yl)-1,3,2-dioxaborolane synthesis
- Product Name:4,4,5,5-tetramethyl-2-(thieno[3,2-b]thiophen-2-yl)-1,3,2-dioxaborolane
- CAS Number:1004784-50-2
- Molecular formula:C12H15BO2S2
- Molecular Weight:266.19
![Thieno[3,2-b]thiophene](/CAS/GIF/251-41-2.gif)
251-41-2
272 suppliers
$8.00/250mg
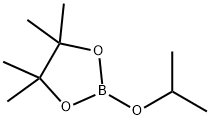
61676-62-8
310 suppliers
$11.19/5G
![4,4,5,5-tetramethyl-2-(thieno[3,2-b]thiophen-2-yl)-1,3,2-dioxaborolane](/CAS/20180703/GIF/1004784-50-2.gif)
1004784-50-2
27 suppliers
inquiry
Yield:1004784-50-2 85.4%
Reaction Conditions:
Stage #1: thieno[3,2-b]thiophenewith n-butyllithium in tetrahydrofuran;hexane at -70; for 1 h;Inert atmosphere;
Stage #2: 2-Isopropoxy-4,4,5,5-tetramethyl-1,3,2-dioxaborolane in tetrahydrofuran;hexane at -70 - 20; for 16 h;Inert atmosphere;
Steps:
41.1 Step 1: 4,4,5,5-tetramethyl-2-(thieno[3,2-b]thiophen-2-yl)-1,3,2-dioxaborolane (149)
[0602] To a solution of 148 (5 g, 35.65 mmol) in THF (70 mL) was added n-BuLi (1.6 M in hexanes, 23.4 mL, 37.4 mmol) at -70 °C dropwise for 30 min with stirring under N2 atmosphere. After addition, the reaction was stirred at the same temperature for 30 min, followed by the addition of 2-isopropoxy-4,4,5,5-tetramethyl-1,3,2-dioxaborolane (7.29 g, 39.2 mmol). The reaction was allowed to warm to room temperature and stirred for 16 hrs. After quenched with saturated aq. NH4Cl solution at 0 oC, the mixture was extracted with EtOAc twice. The combined organic layers are washed with brine, dried over anhydrous Na2SO4 and concentrated to dryness to give the title compound (8.1 g, 85.4% yield) as light green solid.
References:
WO2017/35357,2017,A1 Location in patent:Paragraph 0416; 0602
![Thieno[3,2-b]thiophene](/CAS/GIF/251-41-2.gif)
251-41-2
272 suppliers
$8.00/250mg
76-09-5
376 suppliers
$10.00/1g
![4,4,5,5-tetramethyl-2-(thieno[3,2-b]thiophen-2-yl)-1,3,2-dioxaborolane](/CAS/20180703/GIF/1004784-50-2.gif)
1004784-50-2
27 suppliers
inquiry
![Thieno[3,2-b]thiophene](/CAS/GIF/251-41-2.gif)
251-41-2
272 suppliers
$8.00/250mg
25015-63-8
216 suppliers
$22.39/5G
![4,4,5,5-tetramethyl-2-(thieno[3,2-b]thiophen-2-yl)-1,3,2-dioxaborolane](/CAS/20180703/GIF/1004784-50-2.gif)
1004784-50-2
27 suppliers
inquiry
![Thieno[3,2-b]thiophene](/CAS/GIF/251-41-2.gif)
251-41-2
272 suppliers
$8.00/250mg

76-09-5
376 suppliers
$10.00/1g
10294-34-5
257 suppliers
$26.00/25ML
![4,4,5,5-tetramethyl-2-(thieno[3,2-b]thiophen-2-yl)-1,3,2-dioxaborolane](/CAS/20180703/GIF/1004784-50-2.gif)
1004784-50-2
27 suppliers
inquiry
![Thieno[3,2-b]thiophene](/CAS/GIF/251-41-2.gif)
251-41-2
272 suppliers
$8.00/250mg
![4,4,5,5-TETRAMETHYL-[1,3,2]DIOXABOROLAN-2-OL](/CAS/GIF/25240-59-9.gif)
25240-59-9
42 suppliers
inquiry
![4,4,5,5-tetramethyl-2-(thieno[3,2-b]thiophen-2-yl)-1,3,2-dioxaborolane](/CAS/20180703/GIF/1004784-50-2.gif)
1004784-50-2
27 suppliers
inquiry