
4-CHLORO-5-IODO-1-(TRIISOPROPYLSILYL)-1H-PYRROLO[2,3-B]PYRIDINE synthesis
- Product Name:4-CHLORO-5-IODO-1-(TRIISOPROPYLSILYL)-1H-PYRROLO[2,3-B]PYRIDINE
- CAS Number:1015609-83-2
- Molecular formula:C16H24ClIN2Si
- Molecular Weight:434.82
![1H-Pyrrolo[2,3-b]pyridine, 4-chloro-1-[tris(1-methylethyl)silyl]-](/CAS/GIF/651744-48-8.gif)
651744-48-8
66 suppliers
$225.00/1g
![4-CHLORO-5-IODO-1-(TRIISOPROPYLSILYL)-1H-PYRROLO[2,3-B]PYRIDINE](/CAS/GIF/1015609-83-2.gif)
1015609-83-2
15 suppliers
$155.00/50mg
Yield:1015609-83-2 53%
Reaction Conditions:
Stage #1: 4-chloro-1-triisopropylsilanyl-1H-pyrrolo[2,3-b]pyridinewith sec.-butyllithium in tetrahydrofuran at -78; for 1 h;
Stage #2: with iodine in tetrahydrofuran at -78 - 0; for 0.5 h;
Steps:
3.5 Synthesis of 5
53 ml of sec-BuLi are added dropwise over the course of 30 min to a solution of 11 g of intermediate 4 in 250 ml of THF at -78° C. After 1 h, 18 g of iodine are added dropwise over the course of 30 min. While the batch warms to 0° C., a suspension forms. The mixture is worked up using saturated ammonium chloride solution and extracted with ethyl acetate. The combined organic phases are washed with water and saturated NaCL solution and dried over sodium sulfate, giving 5.25 g (53%) of the product 4-chloro-5-iodo-1-triisopropylsilanyl-1 H-pyrrolo[2,3-b]pyridine as colourless solid; LCMS: (method A) 435.0 (M+H), RT. 7.98 min, 96.9% (max). 1H NMR 400 MHz, DMSO-d6: δ [ppm] 8.52 (s, 1H), 7.57 (d, J=3.52 Hz, 1H), 6.67 (d, J=3.48 Hz, 1H), 1.80-1.88 (m, 3H), 1.04 (d, J=7.52 Hz, 18H).
References:
US2015/218155,2015,A1 Location in patent:Paragraph 0319; 0320
7553-56-2
618 suppliers
$19.00/25g
![1H-Pyrrolo[2,3-b]pyridine, 4-chloro-1-[tris(1-methylethyl)silyl]-](/CAS/GIF/651744-48-8.gif)
651744-48-8
66 suppliers
$225.00/1g
![4-CHLORO-5-IODO-1-(TRIISOPROPYLSILYL)-1H-PYRROLO[2,3-B]PYRIDINE](/CAS/GIF/1015609-83-2.gif)
1015609-83-2
15 suppliers
$155.00/50mg
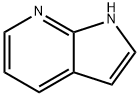
271-63-6
571 suppliers
$9.00/5g
![4-CHLORO-5-IODO-1-(TRIISOPROPYLSILYL)-1H-PYRROLO[2,3-B]PYRIDINE](/CAS/GIF/1015609-83-2.gif)
1015609-83-2
15 suppliers
$155.00/50mg
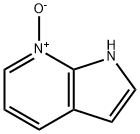
55052-24-9
149 suppliers
$80.00/5G
![4-CHLORO-5-IODO-1-(TRIISOPROPYLSILYL)-1H-PYRROLO[2,3-B]PYRIDINE](/CAS/GIF/1015609-83-2.gif)
1015609-83-2
15 suppliers
$155.00/50mg
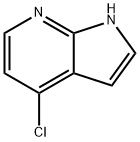
55052-28-3
473 suppliers
$6.00/1g
![4-CHLORO-5-IODO-1-(TRIISOPROPYLSILYL)-1H-PYRROLO[2,3-B]PYRIDINE](/CAS/GIF/1015609-83-2.gif)
1015609-83-2
15 suppliers
$155.00/50mg