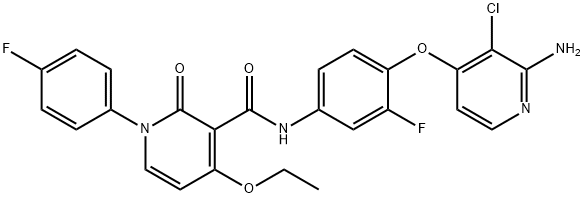
N-[4-[(2-Amino-3-chloropyridin-4-yl)oxy]-3-fluorophenyl]-4-ethoxy-1-(4-fluorophenyl)-2-oxo-1,2-dihydropyridine-3-carboxamide synthesis
- Product Name:N-[4-[(2-Amino-3-chloropyridin-4-yl)oxy]-3-fluorophenyl]-4-ethoxy-1-(4-fluorophenyl)-2-oxo-1,2-dihydropyridine-3-carboxamide
- CAS Number:1025720-94-8
- Molecular formula:C25H19ClF2N4O4
- Molecular Weight:512.89
![2-?Pyridinecarboxamide, 3-?chloro-?4-?[4-?[[[4-?ethoxy-?1-?(4-?fluorophenyl)?-?1,?2-?dihydro-?2-?oxo-?3-?pyridinyl]?carbonyl]?amino]?-?2-?fluorophenoxy]?-](/CAS/20200611/GIF/1025721-14-5.gif)
1025721-14-5
2 suppliers
inquiry
![N-[4-[(2-Amino-3-chloropyridin-4-yl)oxy]-3-fluorophenyl]-4-ethoxy-1-(4-fluorophenyl)-2-oxo-1,2-dihydropyridine-3-carboxamide](/CAS/GIF/1025720-94-8.gif)
1025720-94-8
97 suppliers
$28.00/1unit
Yield:1025720-94-8 74%
Reaction Conditions:
with [bis(acetoxy)iodo]benzene in water;ethyl acetate;acetonitrile at 0 - 20; for 2 h;
Steps:
1.J
To 3-chloro-4-(4-(4-ethoxy-1-(4-fluorophenyl)-2-oxo-1,2-dihydropyridine-3-carboxamido)-2-fluorophenoxy)picolinamide (1.2 g, 2.1 mmol) in ethyl acetate (16 mL), acetonitrile (16 mL), and water (8 mL) at 0° C. was added iodobenzene diacetate (820 mg, 2.6 mmol, Aldrich). After stirring at rt for 2 h, the reaction was filtered to collect the crude product. The solid was washed with additional ethyl acetate. The filtrate was washed with saturated aqueous sodium bicarbonate solution and the organic phase was dried over anhydrous sodium sulfate and concentrated in vacuo. The precipitate and filtrate were combined and purified by flash chromatography on silica gel (2% methanol/chloroform) to give the title compound (810 mg, 74%) as a white solid. 1H NMR (DMSO-d6) δ 10.57 (s, 1H), 7.83-7.79 (m, 2H), 7.67 (d, 1H, J=5.6 Hz), 7.41-7.38 (m, 3H), 7.36-7.22 (m, 3H), 6.44 (d, 1H, J=7.6 Hz), 6.36 (br s, 2H), 5.86 (d, 1H, J=6.0 Hz), 4.18 (q, 2H, J=7.2 Hz), 1.23 (t, 3H, J=7.2 Hz); MS (ESI+) m/z 513.09 (M+H)+.
References:
US2008/114033,2008,A1 Location in patent:Page/Page column 9
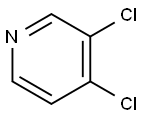
55934-00-4
172 suppliers
$10.00/1g
![N-[4-[(2-Amino-3-chloropyridin-4-yl)oxy]-3-fluorophenyl]-4-ethoxy-1-(4-fluorophenyl)-2-oxo-1,2-dihydropyridine-3-carboxamide](/CAS/GIF/1025720-94-8.gif)
1025720-94-8
97 suppliers
$28.00/1unit
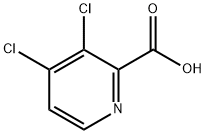
959578-03-1
58 suppliers
$50.00/100mg
![N-[4-[(2-Amino-3-chloropyridin-4-yl)oxy]-3-fluorophenyl]-4-ethoxy-1-(4-fluorophenyl)-2-oxo-1,2-dihydropyridine-3-carboxamide](/CAS/GIF/1025720-94-8.gif)
1025720-94-8
97 suppliers
$28.00/1unit
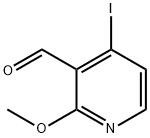
158669-26-2
135 suppliers
$12.00/250mg
![N-[4-[(2-Amino-3-chloropyridin-4-yl)oxy]-3-fluorophenyl]-4-ethoxy-1-(4-fluorophenyl)-2-oxo-1,2-dihydropyridine-3-carboxamide](/CAS/GIF/1025720-94-8.gif)
1025720-94-8
97 suppliers
$28.00/1unit
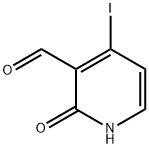
726206-53-7
12 suppliers
inquiry
![N-[4-[(2-Amino-3-chloropyridin-4-yl)oxy]-3-fluorophenyl]-4-ethoxy-1-(4-fluorophenyl)-2-oxo-1,2-dihydropyridine-3-carboxamide](/CAS/GIF/1025720-94-8.gif)
1025720-94-8
97 suppliers
$28.00/1unit