
3-Azabicyclo[3.1.0]hexane-2-carbonitrile, 6,6-dimethyl-, (1R,2S,5S)- synthesis
- Product Name:3-Azabicyclo[3.1.0]hexane-2-carbonitrile, 6,6-dimethyl-, (1R,2S,5S)-
- CAS Number:1037559-82-2
- Molecular formula:C8H12N2
- Molecular Weight:136.19
Yield:1037559-82-2 77%
Reaction Conditions:
Stage #1: (1R-cis)-6,6-dimethyl-3-azabicyclo[3.1.0]hexan-2-onewith sodium methoxide;sodium bis(2-methoxyethoxy)aluminium dihydride in tetrahydrofuran;toluene at -10; for 12 h;
Stage #2: sodium cyanidewith sodium D-gluconate;glacial acetic acid in tetrahydrofuran;lithium hydroxide monohydrate;toluene at 0; for 12 h;Temperature;Concentration;
Steps:
19-25 Example 19
11.6g of bicyclic lactam 1a, 1g of sodium methoxide and 30g of tetrahydrofuran were added to the reaction flask, cooled to -10°C, and sodium dihydrobis(2-methoxyethoxy)aluminate was added dropwise at -10°C for 2 hours A toluene solution of (Red-Al) (70% weight concentration, Red-Al mass 11.2 g) was stirred at the same temperature for 10 hours.The reaction mixture was slowly added dropwise to a pre-cooled mixture of 7 g of sodium gluconate, 5 g of sodium cyanide and 100 g of water at 0 °C, and then 2.8 g of acetic acid was added dropwise to the mixed solution at 0 °C, and the mixture was kept stirring for 12 After one hour, 30 g of toluene was added, stirred, and left to stand for liquid separation.The organic layer was washed twice with 100 g of an aqueous sodium hydroxide solution (40% concentration), and then once with brine.The obtained organic layer was distilled to remove the solvent under reduced pressure, and the residue was distilled under high vacuum (specifically) to obtain 9.7 g of bicyclic cyanopyrrole 4.The yield was 77%, the purity was 99.2%, and the dr value was 99:1.
References:
CN115010647,2022,A Location in patent:Paragraph 0076-0083
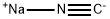
773837-37-9
0 suppliers
inquiry
![6,6-DiMethyl-3-azabicyclo[3.1.0]hexane Boceprevir Key interMediate](/CAS/GIF/943516-54-9.gif)
943516-54-9
222 suppliers
$17.00/100mg
![3-Azabicyclo[3.1.0]hexane-2-carbonitrile, 6,6-dimethyl-, (1R,2S,5S)-](/CAS/20210111/GIF/1037559-82-2.gif)
1037559-82-2
3 suppliers
inquiry
1037559-68-4
3 suppliers
inquiry
![3-Azabicyclo[3.1.0]hexane-2-carbonitrile, 6,6-dimethyl-, (1R,2S,5S)-](/CAS/20210111/GIF/1037559-82-2.gif)
1037559-82-2
3 suppliers
inquiry
67911-21-1
331 suppliers
$6.00/1g
![3-Azabicyclo[3.1.0]hexane-2-carbonitrile, 6,6-dimethyl-, (1R,2S,5S)-](/CAS/20210111/GIF/1037559-82-2.gif)
1037559-82-2
3 suppliers
inquiry
![3-Azabicyclo[3.1.0]hexan-2-one,6,6-dimethyl-,(1R-cis)-(9CI)](/CAS/GIF/159172-92-6.gif)
![3-Azabicyclo[3.1.0]hex-2-ene, 6,6-dimethyl-, (1R,5S)-](/CAS/20200611/GIF/1037559-71-9.gif)