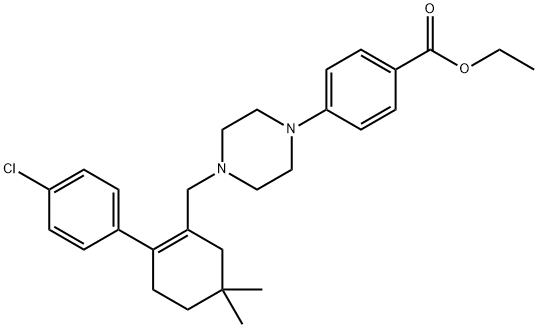
4-[4-[[2-(4-Chlorophenyl)-5,5-dimethyl-1-cyclohexen-1-yl]methyl]-1-piperazinyl]benzoic Acid Ethyl Ester synthesis
- Product Name:4-[4-[[2-(4-Chlorophenyl)-5,5-dimethyl-1-cyclohexen-1-yl]methyl]-1-piperazinyl]benzoic Acid Ethyl Ester
- CAS Number:1065604-70-7
- Molecular formula:C28H35ClN2O2
- Molecular Weight:467.04
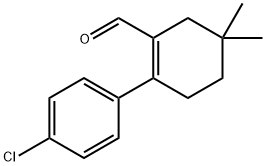
![Benzene, 1-[2-(broMoMethyl)-4,4-diMethyl-1-cyclohexen-1-yl]-4-chloro-](/CAS/GIF/1027345-22-7.gif)
1027345-22-7
12 suppliers
inquiry

80518-57-6
114 suppliers
$11.00/1g
![4-[4-[[2-(4-Chlorophenyl)-5,5-dimethyl-1-cyclohexen-1-yl]methyl]-1-piperazinyl]benzoic Acid Ethyl Ester](/CAS2/GIF/1065604-70-7.gif)
1065604-70-7
11 suppliers
$120.00/10mg
Yield:1065604-70-7 Ca. 700 mg
Reaction Conditions:
with potassium carbonate in dimethyl sulfoxide at 100; for 2 h;
Steps:
1.3.E
To a solution of 3.5 (500.00 mg, 1 .59 mmol) in DMSO (5.00 mL) were added ethyl 4-piperazin-1 -ylbenzoate (372.52 mg, 1 .59 mmol) and K2003 (439.51 mg, 3.18 mmol). The reaction mixture wasstirred at 100 00 for 2 h and then poured into H20 (50 mL). The resulting mixture was extracted with EtOAc (2 x 40 mL) and the combined organic phases were dried over anhydrous Mg504, filtered and concentrated. The residue was washed with petroleum ether (2x5 mL), and dried under reduced pressure to give 3.6 (700.00 mg, 1 .50 mmol, 94% yield), which was pure enough to be used in the next step without further purification. MS (ESI) m/z = 468 [M÷H]. 1H NMR (400 M, ODd3), ? 7.90 (m, 2H),7.27 (m, 2H), 7.00 (m, 2H), 6.81 (m, 2H), 4.31 (q, 2H, J= 6.8 Hz), 3.26 (m, 4H), 2.80 (m, 2H), 2.35 (m,4H), 2.25 (m, 2H), 2.02 (m, 2H), 1.46 (m, 2H), 1.36 (t, 3H, J = 6.8 Hz), 0.99 (s, 9H).
References:
WO2017/101851,2017,A1 Location in patent:Paragraph 0140; 0146
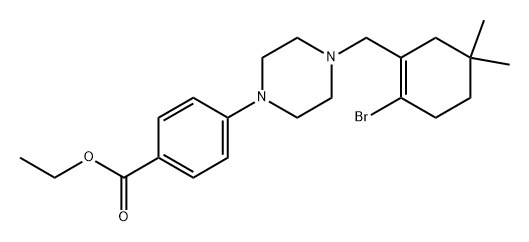
1065604-58-1
2 suppliers
inquiry
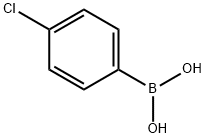
1679-18-1
588 suppliers
$10.00/1g
![4-[4-[[2-(4-Chlorophenyl)-5,5-dimethyl-1-cyclohexen-1-yl]methyl]-1-piperazinyl]benzoic Acid Ethyl Ester](/CAS2/GIF/1065604-70-7.gif)
1065604-70-7
11 suppliers
$120.00/10mg
1202186-71-7
13 suppliers
inquiry

80518-57-6
114 suppliers
$11.00/1g
![4-[4-[[2-(4-Chlorophenyl)-5,5-dimethyl-1-cyclohexen-1-yl]methyl]-1-piperazinyl]benzoic Acid Ethyl Ester](/CAS2/GIF/1065604-70-7.gif)
1065604-70-7
11 suppliers
$120.00/10mg
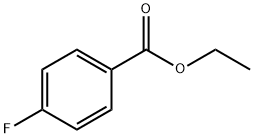
451-46-7
287 suppliers
$5.00/5g
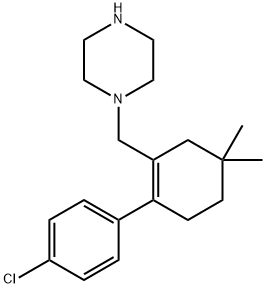
1228838-29-6
10 suppliers
inquiry
![4-[4-[[2-(4-Chlorophenyl)-5,5-dimethyl-1-cyclohexen-1-yl]methyl]-1-piperazinyl]benzoic Acid Ethyl Ester](/CAS2/GIF/1065604-70-7.gif)
1065604-70-7
11 suppliers
$120.00/10mg
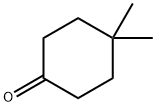
4255-62-3
201 suppliers
$10.00/1g
![4-[4-[[2-(4-Chlorophenyl)-5,5-dimethyl-1-cyclohexen-1-yl]methyl]-1-piperazinyl]benzoic Acid Ethyl Ester](/CAS2/GIF/1065604-70-7.gif)
1065604-70-7
11 suppliers
$120.00/10mg