
5-(1-methyl-1H-pyrazol-5-yl)-2-Pyrimidinecarboxylic acid synthesis
- Product Name:5-(1-methyl-1H-pyrazol-5-yl)-2-Pyrimidinecarboxylic acid
- CAS Number:1067612-22-9
- Molecular formula:C9H8N4O2
- Molecular Weight:204.19
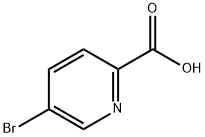
30766-11-1
393 suppliers
$3.00/1g
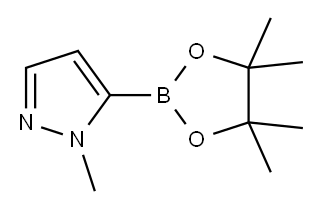
847818-74-0
268 suppliers
$6.00/1g

1067612-22-9
2 suppliers
inquiry
Yield:1067612-22-9 36%
Reaction Conditions:
with potassium carbonate;tetrakis(triphenylphosphine) palladium(0) in 1,4-dioxane;water at 80; for 12 h;Sealed tube;
Steps:
3.a
Example 3Preparation of /V-(2-amino-1-benzylethyl)-5-(1-methyl-1 H-pyrazol-5-yl)pyrimidine-2- carboxamidea) 5-(1-methyl-1 H-pyrazol-5-yl)pyrimidine-2-carboxylic acidTo a solution of 1-methyl-5-(4,4,5,5-tetramethyl-1 ,3,2-dioxaborolan-2-yl)-1 H- pyrazole (1.35 g, 6.5 mmol) in dioxane/H2O (5:1 , 59 ml.) was added K2CO3 (2.8g, 20.3 mmol), tetrakistriphenylphosphine Pd(O) (340 mg, 0.3 mmol), and 5-bromo-2- pyrimidinecarboxylic acid (1.2 g, 5.9 mmol). The reaction mixture was heated to 80° C in a sealed tube for 12h. The reaction solution was poured onto H2O (100 ml.) and extracted with DCM. The organics were dried (Na2SO4), concentrated under vacuum to give the title compound (0.9 g, 36%) as a tan solid: LC-MS (ES) m/z = 437 (M+H)+.
References:
WO2008/121786,2008,A1 Location in patent:Page/Page column 47